Abstract.
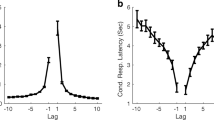
We introduce a sensitive test of memory effects in successive events. The test consists of a combination K of binary correlations at successive times. K decays monotonically from K = 1 for uncorrelated events as a Markov process. For a monotonic memory fading, \( K<1\) always. Here we report evidence of a \( K>1\) temporal window in cognitive tasks consisting of the visual identification of the front face of the Necker cube after a previous presentation of the same. We speculate that memory effects provide a temporal window with \( K>1\) and this experiment could be a possible first step towards a better comprehension of this phenomenon. The \( K>1\) behaviour is maximal at an inter-measurement time \( \tau\) around 2s with inter-subject differences. The \( K>1\) persists over a time window of 1s around \( \tau\); outside this window the \(K<1\) behaviour is recovered. The universal occurrence of a \( K>1\) window in pairs of successive perceptions suggests that, at variance with single visual stimuli eliciting a suitable response, a pair of stimuli shortly separated in time displays mutual correlations.
Similar content being viewed by others
References
Gerald M. Long, Thomas C. Toppino, Psychol. Bull. 130, 748 (2004)
L.A. Necker, London Edinburgh Philos. Mag. J. Sci. 1, 329 (1832)
A.J. Leggett, A. Garg, Phys. Rev. Lett. 54, 857 (1985)
Clive Emary, Neill Lambert, Franco Nori, Rep. Prog. Phys. 77, 016001 (2014)
D.H. Brainard, Spat. Vis. 10, 433 (1997)
D.G. Pelli, Spat. Vis. 10, 437 (1997)
M. Kleiner, D. Brainard, D. Pelli, A. Ingling, R. Murray, C. Broussard, Perception 36, 1 (2007)
Ming Meng, Frank Tong, J. Vis. 4, 2 (2004)
David A. Leopold, Melanie Wilke, Alexander Maier, Nikos K. Logothetis, Nat. Neurosci. 5, 605 (2002)
Joel Pearson, Jan Brascamp, Trends Cogn. Sci. 12, 334 (2008)
J.W. Brascamp, J. Pearson, R. Blake, A.V. Van Den Berg, J. Vis. 9, 3 (2009)
Naoki Kogo, Lore Hermans, David Stuer, Raymond van Ee, Johan Wagemans, Vis. Res. 106, 7 (2015)
P. Neri, M.C. Morrone, D.C. Burr, Nature 395, 894 (1998)
G. Wyszecki, W.S. Stiles, Color science (John Wiley & Sons, New York, 1982)
R. Arrighi, F.T. Arecchi, A. Farini, C. Gheri, Cogn. Proc. 10, S95 (2009)
A. Borsellino, A. Marco, A. Allazetta, S. Rinesi, B. Bartolini, Biol. Cybern. 10, 139 (1972)
D.A. Leopold, N.K. Logothetis, Trends Cogn. Sci. 3, 254 (1999)
H. Atmanspacher, T. Filk, H. Römer, Biol. Cybern. 90, 33 (2004)
E. Pöppel, Trends Cogn. Sci. 1, 56 (1997)
C. Koch, The quest for consciousness: A neuroscientific approach (Roberts & Co, 2004)
Jason Fischer, David Whitney, Nat. Neurosci. 17, 738 (2014)
Guido Marco Cicchini, Giovanni Anobile, David C. Burr, Proc. Natl. Acad. Sci. U.S.A. 111, 7867 (2014)
Guido Gigante, Maurizio Mattia, Jochen Braun, Paolo Del Giudice, PLoS Comput. Biol. 5, e1000430 (2009)
F.T. Arecchi, Eur. Phys. J. ST 146, 205 (2007)
T.L. Griffiths, C. Kemp, J.B. Tenenbaum, Bayesian models of cognition, in Cambridge handbook of computational cognitive modeling (Cambridge University Press, 2008) pp. 59--100
J.P. Lachaux, E. Rodriguez, J. Martinerie, F.J. Varela et al., Hum. Brain Mapp. 8, 194 (1999)
F.T. Arecchi, J. Psychophysiol. 24, 141 (2010)
Eugenio Rodriguez, Nathalie George, Jean-Philippe Lachaux, Jacques Martinerie, Bernard Renault, Francisco J. Varela, Nature 397, 430 (1999)
K.P. Körding, D.M. Wolpert, Trends Cogn. Sci. 10, 319 (2006)
E. Poppel, Acta Neurobiol. Exp. 64, 295 (2004)
F.T. Arecchi, Nonlinear Dyn. Psychol. Life Sci. 15, 359 (2011)
S. Dehaene, C. Sergent, J.P. Changeux, Proc. Natl. Acad. Sci. U.S.A. 100, 8520 (2003)
E. Pöppel, Philos. Trans. R. Soc. B: Biol. Sci. 364, 1887 (2009)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Arecchi, F.T., Farini, A. & Megna, N. A test of multiple correlation temporal window characteristic of non-Markov processes. Eur. Phys. J. Plus 131, 50 (2016). https://doi.org/10.1140/epjp/i2016-16050-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjp/i2016-16050-6