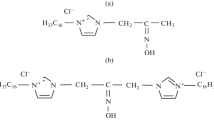
Abstract.
The shear behavior and the normal interaction between mica surfaces covered by surfactant or surfactant-polymer mixtures were studied with a Surface Forces Apparatus (SFA) nanotribometer. If the surfaces are compressed while fully immersed in an aqueous surfactant solution that adsorbs in the form of flat bilayers, hemifusion can be induced. When the hemifused surfaces are subject to shear, at least five different dynamic regimes can be recognized. The general behavior may be described by a model based on the kinetics of formation and rupture of adhesive bonds between the shearing surfaces, with an additional viscous term. Once the adsorbed surfactant layer is decorated with physigrafted copolymers, the number of sliding regimes may be reduced to only one, in which the shear stress increases sublinearly with the driving velocity. The adhesion energy and the resistance to hemifusion of the adsorbed surfactant-polymer layers are also strongly modified as the grafting density increases.
Similar content being viewed by others
References
P. Richetti, C. Drummond, J. Israelachvili, M. In, R. Zana, Europhys. Lett. 55, 653 (2001).
C. Drummond, J. Israelachvili, P. Richetti, Phys. Rev. E 67, 066110 (2003).
R.M. Pashley, J.N. Israelachvili, Colloids Surf. 2, 169 (1981).
J. Israelachvili, Intermolecular and Surface Forces (Academic, San Diego, 1992).
C.A. Helm, J.N. Israelachvili, P.M. McGuiggan, Biochemistry 31, 1794 (1992).
A. Schallamach, Wear 6, 375 (1963); 17, 301 (1971).
Y.B. Chernyak, A.I. Leonov, Wear 108, 105 (1986).
C. Robelin, F.P. Duval, P. Richetti, G.G. Warr, Langmuir 18, 1634 (2002).
M. In, in Reactions and Synthesis in Surfactant Systems, Surfactant Series, edited by J. Tetxer (M. Dekker Inc., 2001) pp. 59-110.
R. Zana, H. Levy, D. Papoutsi, G. Beinert, Langmuir 11, 3694 (1995).
A. Homola, J.N. Israelachvili, M.L. Gee, P. McGuiggan, J. Tribol. 111, 675 (1989).
J.N. Israelachvili, J. Colloid Interface Sci. 44, 259 (1973).
G. Luengo, F.-J. Schmitt, R. Hill, J. Israelachvili, Macromolecules 30, 2482 (1997).
A. Blom, G. Warr, private communication.
U. Raviv, J. Frey, R. Sak, P. Laurat, R. Tadmor, J. Klein, Langmuir 18, 7482 (2002).
C.A. Helm, J.N. Israelachvili, P.M. McGuiggan, Science 246, 919 (1989).
E. Barthel, Colloids Surf. A 149, 99 (1999).
W.-H. Hu, G.A. Carson, S. Granick, Phys. Rev. Lett. 66, 2758 (1991).
S. Yamada, Langmuir 19, 7399 (2003).
L. Bureau, Léger, L., unpublished (2003).
A. Subbotin, A. Semenov, E. Manias, G. Hadziioannou, G. Brinke, Macromolecules 28, 3898 (1995).
J.N. Bright, D.R. Williams, Langmuir 15, 3836 (1999).
Author information
Authors and Affiliations
Corresponding author
Additional information
Received: 4 March 2004, Published online: 13 October 2004
PACS:
46.55. + d Tribology and mechanical contacts - 81.40.Pq Friction, lubrication, and wear
Rights and permissions
About this article
Cite this article
Drummond, C., In, M. & Richetti, P. Behavior of adhesive boundary lubricated surfaces under shear: Effect of grafted diblock copolymers. Eur. Phys. J. E 15, 159–165 (2004). https://doi.org/10.1140/epje/i2004-10043-y
Issue Date:
DOI: https://doi.org/10.1140/epje/i2004-10043-y