Abstract.
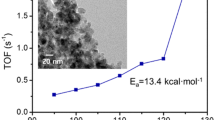
We have prepared titania aerogel and titania-coated silica aerogel incorporating thiol-capped Au nanoparticles. Both composite materials showed high CO oxidation activity after they were calcined at 673 K. Compositional and morphological changes driven by calcination were evaluated with thermogravimetry and X-ray diffractometry. From the results, it was suggested that the nanoparticles transformed from a faulted to a near-regular FCC structure presumably in concert with the formation of firm contacts between the nanoparticles and the gel substrates. While the diameters of the Au particles in the titania aerogel considerably increased upon calcination, those in the titania-coated silica aerogel were almost unchanged. As a consequence, the latter composite aerogel showed higher activity for oxidation of CO.
Similar content being viewed by others
References
M. Haruta, Chem. Record 3, 75 (2003)
B.S. Uphade, T. Akita, T. Nakamura, M. Haruta, J. Catal. 209, 331 (2002)
Q. Fu, H. Saltsburg, M. Flytzani-Stephanopoulos, Science 301, 935 (2003)
L.D. Socaciu, J. Hagen, T.M. Bernhardt, L. Woeste, U. Heiz, H. Hakkinen, U. Landman, J. Am. Chem. Soc. 125, 10437 (2003)
A. Sanchez, S. Abbet, U. Heiz, W.D. Schneider, H. Hakkinen, R.N. Barnett, U. Landman, J. Phys. Chem. A 103, 9573 (1999)
M. Valden, X. Lai, D.W. Goodman, Science 281, 1647 (1998)
Y. Tai, M. Watanabe, K. Kaneko, S. Tanemura, T. Miki, J. Murakami, K. Tajiri, Adv. Mater. 13, 1611 (2001)
Y. Tai, J. Murakami, K. Tajiri, F. Ohashi, M. Dat\(\acute{\rm e}\), S. Tsubota, Appl. Catal. A-Gen. 268, 183 (2004)
G. Dagan, M. Tomkiewics, J. Phys. Chem. 97, 12651 (1993)
K. Tajiri, Y. Tai, Proceedings of the 20th International Japan-Korea Seminar on Ceramics (2003), p. 561
S. Srinivasan, A.K. Datye, M. Hampden-Smith, I.E. Wachs, G. Deo, J.M. Jehng, A.M. Turek, C.H.F. Peden, J. Catal. 131, 260 (1991)
M. Brust, M. Walker, D. Bethell, D.J. Schiffrin, R. Whyman, J. Chem. Soc. Chem. Commun. 801 (1994)
K. Ruth, M. Hayes, R. Burch, S. Tsubota, M. Haruta, Appl. Catal. B: Environ. 24, L133 (2000)
C.J. Brinker, G.W. Scherer, Sol-gel Science (Academic Press, 1989), p. 551
R.L. Whetten, J.T. Khoury, M.M. Alvarez, S. Murthy, I. Vezmar, Z.L. Wang, P.W. Stephens, C.L. Cleaveland, W.D. Luedtke, U. Landman, Adv. Mater. 8, 428 (1996)
J.J. Pietron, R.M. Stroud, D.R. Rolison, Nano Lett. 2, 545 (2002)
T.G. Schaaff, M.N. Shafigullin, J.T. Khoury, I. Vezmar, R.L. Whetten, W.G. Cullen, P.N. First, C. Gutierrez-Wing, J. Ascensio, M. Jose-Yacaman, J. Phys. Chem. B 101, 7885 (1997)
T. Yonezawa, K. Yasui, N. Kimizuka, Langmuir 17, 271 (2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tai, Y., Ochi, Y., Ohashi, F. et al. Oxidation activity of Au nanoparticles on aerogel supports. Eur. Phys. J. D 34, 125–128 (2005). https://doi.org/10.1140/epjd/e2005-00132-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1140/epjd/e2005-00132-7