Abstract.
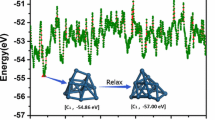
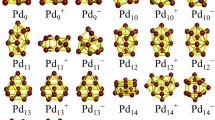
Using molecular dynamics and thermal quenching simulation techniques, and the basin-hopping Monte Carlo algorithm we have studied the global minima and energetics of free Pt N clusters in the size range of N = 22-56. The clusters have been described by the Voter and Chen version of an embedded-atom model, which is derived by fitting to experimental data of both the diatomic molecule and bulk platinum simultaneously. A comparison between the two search techniques has been performed and it is found that the basin-hopping algorithm is more efficient than a molecular dynamics minimization approach in the investigation of the global minima. The results show that the global minima of the Pt clusters have structures based on either octahedral, decahedral or icosahedral packing. Some of the icosahedral global minima do not have a central atom. The 54-atom icosahedron without a central atom is found to be more stable than the 55-atom complete icosahedron. The resulting structures have been compared with the previous theoretical calculations.
Similar content being viewed by others
References
A. Sebetci, Z.B. Güvenç, Surf. Sci. 525, 66 (2003); and references therein
D.J. Wales, J.P.K. Doye, J. Phys. Chem. A 101, 5111 (1997)
Z. Li, H.A. Scheraga, Proc. Natl. Acad. Sci. USA 84, 6611 (1987)
M. Karabacak, S. Özçelik, Z.B. Güvenç, Surf. Sci. 532-535, 306 (2003)
S. Özçelik, Z.B. Güvenç, Surf. Sci. 532-535, 312 (2003)
R.H. Leary, J. Glob. Opt. 18, 367 (2000)
J.P.K. Doye, D.J. Wales, New J. Chem., 733 (1998)
C. Massen, T.V. Mortimer-Jones, R.L. Johnston, J. Chem. Soc., Dalton Trans. 23, 4375 (2002)
F. Cleri, V. Rosato, Phys. Rev. B 48, 22 (1993)
J.P.K. Doye, M.A. Miller, D.J. Wales, J. Chem. Phys. 111, 8417 (1999)
R.H. Byrd, P. Lu, J. Nocedal, C. Zhu, SIAM J. Sci. Comput. 16, 1190 (1995)
http://www-wales.ch.cam.ac.uk/software.html
J.P.K. Doye, D.J. Wales, R.S. Berry, J. Chem. Phys. 103, 4234 (1995)
J.P.K. Doye, D.J. Wales, J. Chem. Soc., Faraday Trans. 93, 4233 (1997)
J.A. Northby, J. Xie, D.L. Freeman, J.D. Doll, Z. Phys. D 12, 69 (1989)
J.W. Lee, G.D. Stein, J. Phys. Chem. 91, 2450 (1987)
K. Clemenger, Phys. Rev. B 32, 1359 (1985)
Y.J. Lee, E.K. Lee, S. Kim, Pyhs. Rev. Lett. 86, 999 (2001)
A.L. Mackay, Acta Crystallogr. 15, 916 (1962)
N.T. Wilson, R.L. Johnston, Eur. Phys. J. D 12, 161 (2000)
J. Uppenbrink, D.J. Wales, J. Chem. Phys. 96, 8520 (1992)
M. Andersson, A. Rosen, J. Chem. Phys. 117, 7051 (2002)
E.K. Parks, L. Zhu, J. Ho, S.J. Riley, J. Chem. Phys. 100, 7206 (1994)
E.K. Parks, G.C. Nieman, K.P. Kerns, S.J. Riley, J. Chem. Phys. 108, 3731 (1998)
E.K. Parks, S.J. Riley, Z. Phys. D 33, 59 (1995)
E.K. Parks, B.J. Winter, T.D. Klots, S.J. Riley, J. Chem. Phys. 94, 1882 (1991)
R.L. Johnston, Atomic and Molecular Clusters (Taylor and Francis, London, 2002)
N.T. Wilson, R.L. Johnston, Phys. Chem. Chem. Phys. 4, 4168 (2002)
http://brian.ch.cam.ac.uk/CCD.html
Author information
Authors and Affiliations
Corresponding author
Additional information
Received: 30 March 2004, Published online: 18 May 2004
PACS:
36.40.-c Atomic and molecular clusters - 61.46. + w Nanoscale materials: clusters, nanoparticles, nanotubes, and nanocrystals
Rights and permissions
About this article
Cite this article
Sebetci, A., Güvenç, Z.B. Global minima for free \(\mathsf{Pt_{N}}\) clusters (\(\mathsf{N = 22{-}56}\)): a comparison between the searches with a molecular dynamics approach and a basin-hopping algorithm. Eur. Phys. J. D 30, 71–79 (2004). https://doi.org/10.1140/epjd/e2004-00072-8
Issue Date:
DOI: https://doi.org/10.1140/epjd/e2004-00072-8