Abstract
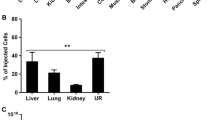
Human carcinoma A431 cells were subcutaneously injected into nude mice at points remote from each other. One of the two xenografts developed afterwards was used for the treatment with a recombinant vaccinia virus, while the other xenograft served as an artificial metastasis. We used the attenuated recombinant vaccinia virus (VACV) VVdGF-GFP2 of the L-IVP strain (GenBank accession number KP233807) with the deletion of two virulence genes, i.e., the genes of the virus growth factor and thymidine kinase, with the gene for the green fluorescent protein (GFP2) inserted in the place of the latter. The treatment was performed by a single intratumoral injection of recombinant VACV at a dose of 107 PFU/mouse. VACV was detected in cells of the artificial metastasis as early as two days after infection. After eight days, the virus concentration was comparable with that in the infected tumor (~109 PFU/mL). Electron microscopy revealed selective replication of the recombinant virus in the tumor cells. The targeted accumulation of GFP2 in both tumor and metastasis was shown in the UV images of the mice obtained using the In-vivo Multispectral Imaging System (Bruker, Germany). The complete destruction of the tumor was detected after 12 days, and that of metastasis, after 20 days after the injection of VVdGF-GFP2. The destruction process was accompanied by pronounced edema and leukocyte infiltration of the tumor tissue. The recombinant virus induced a significant reduction in the sizes of the tumor and metastasis. By the end of the experiment (35 days), the xenografts in the control mice were 10 times larger than those in the treated mice (5000 vs 500 mm3). Our study has shown that the attenuated VACV administered by the peripheral route can not only destroy the primary tumor but also has a distinct antimetastatic effect.
Similar content being viewed by others
References
Breitbach, C., Burke, J., Jonker, D., Stephenson, J., Haas, A., Chow, L., Nieva, J., Hwang, T., Moon, A., Thorne, S., Pelusio, A., LeBoeuf, F., Burns, J., Evgin, L., De Silva, N., et al., Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans, Nature, 2011, vol. 477, pp. 99–102.
Breitbach, C.J., Thorne, S.H., Bell, J.C., and Kirn, D.H., Targeted and armed oncolytic poxviruses for cancer: The lead example of JX-594, Curr. Pharm. Biotechnol., 2012, vol. 3, pp. 1768–1772.
Breitbach, C., Arulanandam, R., De Silva, N., Thorne, S., Thorne, S., Daneshmand, M., Moon, A., Burke, J., and Hwang, T., Oncolytic vaccinia virus disrupts tumor-associated vasculature in humans, Cancer Res., 2013, vol. 73, no. 4, pp. 1265–1275.
Cochran, M.A., Puckett, C., and Moss, B., In vitro mutagenesis of the promoter region for a vaccinia virus gene: Evidence for tandem early and late regulatory signals, J. Virol., 1985, vol. 54, no. 1, pp. 30–37.
Haddad, D., Chen, N., Zhang, Q., Chen, C.-H., Yu, Y.A., Gonzalez, L., Aguilar, J., Li, P., Wong, J., Szalay, A.A., and Fong, Y., A novel genetically modified oncolytic vaccinia virus in experimental models is effective against a wide range of human cancers, Ann. Surg. Oncol., 2012, vol. 19, pp. 665–674.
Kochneva, G.V., Urmanov, I.H., Ryabchicova, E.I., Streltsov, V.V., and Serpinsky, O.I., Fine mechanisms of ectromelia virus thymidine kinase-negative mutants avirulence, Virus Res., 1994, vol. 34, pp. 49–61.
Kochneva, G.V., Sivolobova, G.F., Yudina, K.V., Babkin, I.V., Chumakov, P.M., and Netesov, S.V., Oncolytic poxviruses, Mol. Genet. Mikrobiol. Virusol., 2012, vol. 1, pp. 8–15.
Kochneva, G.V., Babkina, I.N., Lupan, T.A., Grazhdantseva, A.A., Yudin, P.V., Sivolobova, G.F., Shvalov, A.N., Popov, E.G., Babkin, I.V., Netesov, S.V., and Chumakov, P.M., Apoptin enhances the oncolytic activity of vaccinia virus in vitro, Mol. Biol. (Moscow, Russ. Fed., Engl. Ed.), 2013, vol. 47, no. 5, pp. 842–852.
Kochneva, G., Zonov, E., Grazhdantseva, A., Unusova, A., Sivolobova, G., Popov, E., Taranov, O., Netesov, S., Chumakov, P., and Ryabchikova, E., Apoptin enhances the oncolytic properties of vaccinia virus and modifies mechanisms of tumor regression, Oncotarget, 2014, vol. 5, no. 22, pp. 11269–11282.
Marennikova, S.S. and Shchelkunov, S.N., Patogennye dlya cheloveka ortopoksvirusy (Orthopoxviruses Pathogenic for Humans), Moscow, 1998.
McCart, A., Bartlett, D., and Moss, B., Combined growth factor-deleted and thymidine kinase-deleted vaccinia virus vector, USPatent 7208313, 2007.
Thorne, S.H., Hwang, T.H., O’Gorman B.E., Bartlett, D.L., Sei, S., Adiata, F., Brown, C., Werier, J., Jo, J.H., Lee, D.E., Wang, Y., Bell, J., and Kirn, D.H., Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963, J. Clin. Invest., 2007, vol. 117, pp. 3350–3358.
Weibel, S., Raab, V., Yu, Y.A., Worschech, A., Wang, E., Marincola, F.M., and Szalay, A.A., Viral-mediated oncolysis is the most critical factor in the late-phase of the tumor regression process upon vaccinia virus infection, BMC Cancer, 2011, vol. 11, pp. 68–74.
Yu, Z., Li, S., Brader, P., Chen, N., Yu, Y.A., Zhang, Q., Szalay, A.A., Fong, Y., and Wong, R.J., Oncolytic vaccinia therapy of squamous cell carcinoma, Mol. Cancer, 2009, vol. 8, pp. 45–53.
Unusova, A.Yu., Zonov, E.V., Kochneva, G.V., and Ryabchikova, E.I., Morphology of human A431 carcinoma xenografts in nude mice, Vestn. NGU, 2014, vol. 12, no. 3, pp. 42–48.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © G.V. Kochneva, A.A. Grazhdantseva, G.F. Sivolobova, A.V. Tkacheva, A.N. Shvalov, A.Yu. Unusova, E.I. Ryabchikova, S.V. Netesov, 2015, published in Vavilovskii Zhurnal Genetiki i Selektsii, 2015, Vol. 19, No. 4, pp. 480–486.
Rights and permissions
About this article
Cite this article
Kochneva, G.V., Grazhdantseva, A.A., Sivolobova, G.F. et al. Model of artificial metastasis of human epidermoid carcinoma A431 in nude mice for the examination of oncolytic activity of the vaccinia virus. Russ J Genet Appl Res 6, 469–476 (2016). https://doi.org/10.1134/S2079059716040109
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2079059716040109