Abstract
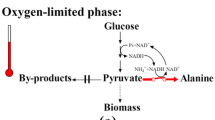
New mutants resistant to L-cycloserine and β-chloro-L-alanine and not described previously were obtained from a parental strain Brevibacterium flavum AA5. Their ability to produce alanine was studied. It was found that the resistance to L-cycloserine did not significantly affect the yield of L-alanine, whereas the resistance to β-chloro-L-alanine of B. flavum GL1 and B. flavum GL18 producers exceeded the initial level of the L-alanine synthesis by 23 and 38%, respectively.
Similar content being viewed by others
References
Beuster, G., Zarse, K., Kaleta, C., et al., Inhibition of alanine aminotransferase in silico and in vivo promotes mitochondrial metabolism to impair malignant growth, J. Biol. Chem., 2011, vol. 25, pp. 22323–22330.
Cornell, N.W., Zuurendonk, P.F., Kerich, M.J., et al., Selective inhibition of alanine aminotransferase and aspartate aminotransferase in rat hepatocytes, Biochem. J., 1984, vol. 220, pp. 707–716.
Dworkin, M., The Prokaryotes: Symbiotic Associations, Biotechnology, Applied Microbiology, 3rd ed., Kumagai, H., Ed., vol. 1, Chapter 3.2, Springer, 2006.
Filipovich, Yu.B., Egorova, T.A., and Sevast’yanova, G.A., Praktikum po obshchei biokhimii (A Practical Course in General Biochemistry), Moscow, 1982.
Gaibakyan, L.D., Avetisova, G.E., Azizyan, A.G., et al., Study of enzymes involved in alanine biosynthesis in Brevibacterium flavum, Biotekhnologiya, 2003, no. 1, pp. 44–48.
Hols, P., Kleerebezem, M., Schanck, A., et al., Conversion of Lactococcus lactis from homolactic to homoalanine fermentation through metabolic engineering, Nature Biotechnol., 1999, vol. 17, pp. 588–592.
Ikeda, M., Amino acid production processes, Adv. Biochem. Engineer./Biotechnol., 2003, vol. 79, pp. 1–35.
Marienhagen, J. and Eggeling, L., Metabolic function of Corynebacterium glutamicum aminotransferases AlaT and AvtA and impact on L-valine production, Appl. Environ. Microbiol., 2008, vol. 74, no. 24, pp. 7457–7462.
Melkonyan, L.H., Avetisova, G.E., Hambardzumyan, A.A., et al., Study of L-glutamate-pyruvate aminotransferase inhibition in wild type strain of Brevibacterium flavum 14067 and L-alanine strain-producer Br. flavum AA5, in Intern. Conf. “State-of the-Art Biotechnology in Armenia and ISTC Contribution,” Armenia, 2008, p. 72.
Miller, J., Experiments in Molecular Genetics, New York: Cold Spring Harbor, 1972.
US Patent No. 5124257, 1992.
US Patent No. 5559016, 1996.
US Patent No. 6627420, 2003.
US Patent No. 20100151449A1, 2010.
Wada, M., Narita, K., and Yokota, A., Alanine production in an H+-ATPase and lactate dehydrogenase-defective mutant of Escherichia coli expressing alanine dehydrogenase, Appl. Microbiol. Biotechnol., 2007, vol. 76, no. 4, pp. 819–825.
Whalen, W.A., Wang, M.D., and Berg, C.M., Beta-chloro-L-alanine inhibition of the Escherichia coli alaninevaline transaminase, J. Bacteriol., 1985, vol. 164, no. 3, pp. 1350–1352.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © G.Ye. Avetisova, L.H. Melkonyan, A.Kh. Chakhalyan, S.Gh. Keleshyan, A.S. Saghyan, 2013, published in Vavilovskii Zhurnal Genetiki i Selektsii, 2013, Vol. 17, No. 3, pp. 430–434.
Rights and permissions
About this article
Cite this article
Avetisova, G.Y., Melkonyan, L.H., Chakhalyan, A.K. et al. Selection of new highly active L-alanine producer strains of Brevibacterium flavum and comparison of their activity in alanine synthesis. Russ J Genet Appl Res 4, 23–26 (2014). https://doi.org/10.1134/S207905971401002X
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S207905971401002X