Abstract
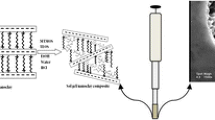
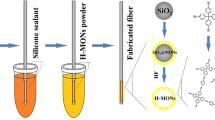
New hybrid organosilica coatings with different amount of alkyl functional groups on a quartz fiber for solid phase microextraction (SPME) have been obtained by the of sol–gel synthesis in the presence of polyethylene glycol (PEG). The structural-sorption properties of the synthesized silica coatings of various compositions have been studied. According to the thermogravimetric analysis, the main thermolysis of the adsorption coatings’ organic layer was observed at temperatures higher than 350°C. The effect of synthesis conditions on the thermal stability of the obtained PEG-based organosilica coating has been studied. It has been established that the textural characteristics of the produced coatings for SPME and their adsorption properties can be controlled using different PEG and silica precursors and the equivalence ratio in the reaction mixture. Good prospects of application of the fabricated coatings for SPME have been demonstrated in paraben extraction and concentration in aqueous solutions at 24°C and pH 3.0–5.5 with their subsequent gas chromatography determination. The suggested technique was characterized by good accuracy and reproducibility (RSD ≤ 2.3%). Comparative studies of the obtained hybrid organosilica coatings for SPME with a commercially available fiber with a bipolar polymer coating—divinylbenzene/carboxen/polydimethylsiloxane—have been conducted.






Similar content being viewed by others
REFERENCES
Soni, M.G., Taylor, S.L., Greenberg, N.A., and Burdock, G.A., Food Chem. Toxicol., 2002, vol. 40, pp. 1335–1373.
Soni, M.G., Carabin, I.G., and Burdock, G.A., Food Chem. Toxicol., 2005, vol. 43, pp. 985–1015.
Elder, R.L., Int. J. Toxicol., 2008, no. 4, Suppl., pp. 1–82.
http://www.ec.europa.eu/health/scientific_committees/ consumer_safety/docs/sccs_o_132.pdf.
Frederiksen, H., Jorgensen, N., and Andersson, A.-M., J. Exposure Sci. Environ. Epidemiol., 2011, vol. 21, pp. 262–333.
Farajzadeh, M.A., Djozan, D.J., and Bakhtiyari, R.F., Talanta, 2010, vol. 81, pp. 1360–1367.
Lokhnauth, J.K. and Snow, N.H., Anal. Chem., 2005, vol. 77, pp. 5938–5946.
Zaitsev, V.N. and Zui, M.F., J. Anal. Chem., 2014, vol. 69, no. 8, pp. 715–728.
https://www.sigmaaldrich.com/technical-documents/ articles/analytical/selecting-spmefibers.html.
Zhang, Zh., Zhu, L., Ma, Yu., Huang, Y., and Li, G., Analyst, 2013, vol. 138, pp. 1156–1166.
Li, Y., Wang, Y., Zhang, J., and Sun, C., Environ. Monit. Assess., 2012, vol. 184, pp. 4345–4353.
Wong, M.Y., Cheng, W.R., Liu, M.H., Tian, W.C., et al., Talanta, 2012, vol. 101, pp. 307–313.
Li, Z., Ma, R., Bai, Sh., Wang, Ch., and Wang, Z., Talanta, 2014, vol. 119, pp. 498–504.
Wang, T., Chen, Y., Ma, J., Hu, M., et al., Anal. Bioanal. Chem., 2014, vol. 406, no. 20, pp. 4955–4963.
Lopez-Darias, J., Anderson, J.L., Pino, V., and Afonso, A.M., Anal. Bioanal. Chem., 2011, vol. 401, pp. 2965–2976.
He, J., Chen, Si., Jiang, Y., Shen, Y., Zhu, J., Wei, H., Zhang, H., and Lu, K., J. Sep. Sci., 2012, vol. 35, pp. 308–314.
Walles, M., Mullett, W.M., and Pawliszyn, J., J. Chromatogr. A, 2004, vol. 1025, p. 85.
Chong, S.L., Wang, D., Hayes, J.D., Wilhite, B.W., et al., Anal. Chem., 1997, vol. 69, p. 3889.
Kumar, A., Gaurav, M.A.K., Tewary, D.K., and Singh, B., Anal. Chim. Acta, 2008, vol. 610, pp. 1–14.
Piri-Moghadam, H., Nazmul, A.Md., and Pawliszyn, J., Anal. Chim. Acta, 2017, vol. 984, pp. 42–65.
Barczak, M., Mcdonagh, C., and Wencel, D., Microchim. Acta, 2016, vol. 183, no. 7, pp. 2085–2109.
Sarafraz-yazdi, A., Piri-Moghadam, H., Es’haghi, Z., and Sepehr, S., Anal. Methods, 2010, vol. 2, pp. 746–752.
Yu, J., Dong, L., Wu, C., Wu, L., and Xing, J., J. Chromatogr. A, 2002, vol. 978, pp. 37–48.
Pragst, F., Anal. Bioanal. Chem., 2007, vol. 388, pp. 1393–1414.
Ghader, M., Shokoufi, N., Es-haghi, A., and Kargosha, K., Anal. Bioanal. Chem., 2017, vol. 409, no. 29, pp. 6739–6744.
Lopez-Darias, J., Pino, V., Meng, Y., Anderson, J.L., et al., J. Chromatogr. A, 2010, vol. 1217, no. 46, pp. 7189–7197.
Diaz-Alvarez, M., Smith, S.P., Spivak, D.A., and Martin-Esteban, A., J. Sep. Sci., 2016, vol. 39, no. 3, pp. 552–558.
Liu, D., Song, N., Cheng, Y., Chen, D., et al., RSC Adv., 2014, vol. 4, pp. 49153–49160.
Chen, Y. and Sidisky, L.M., Anal. Chim. Acta, 2012, vol. 743, pp. 61–68.
Wu, M., Zhang, H., Zeng, B., and Zhao, F., J. Chromatogr. A, 2015, vol. 1384, pp. 22–27.
Es-haghi, A., Hosseininasa, V., and Bagheri, H., Anal. Chim. Acta, 2014, vol. 813, pp. 48–55.
High Performance Liquid Chromatography in Pesticide Residue Analysis, Tuzimski, T. and Sherma, J., Eds., Boca Raton, FL: CRC Press, 2015, p. 582.
Bagheri, H., Piri-moghadam, H., and Ahdi, T., Anal. Chim. Acta, 2012, vol. 742, pp. 45–53.
Infrared and Raman Characteristic Group Frequencies: Tables and Charts, Socrates, G., Ed., Chichester: John Wiley and Sons, 2001.
Lisichkin, G.V., Fadeev, A.Yu., Serdan, A.A., and Nesterenko, P.N., Khimiya privitykh poverkhnostnykh soedinenii (Chemistry of Grafted Surface Compounds), Lisichkin, G.V., Ed., Moscow: Fizmatlit, 2003.
Blaug, S.M. and Grant, D.E., J. Soc. Cosmet. Chem., 1974, vol. 25, no. 9, pp. 495–506.
Alexander, K.S., Laprade, B., Mauger, J.W., and Paruta, A.N., J. Pharm. Sci., 1978, vol. 67, no. 5, pp. 624–627.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by D. Marinin
Rights and permissions
About this article
Cite this article
Shnayder, B.A., Levchyk, V.M., Zui, M.F. et al. Hybrid Organosilica Coatings for Solid Phase Microextraction: Highly Efficient Adsorbents for Determination of Trace Parabens. Prot Met Phys Chem Surf 55, 657–666 (2019). https://doi.org/10.1134/S2070205119040221
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205119040221