Abstract
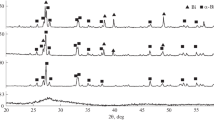
A method of formation of film coatings of titanium dioxide doped by bismuth ions (Bi3+) is developed on the basis of sol–gel synthesis and used to form film coatings of titanium dioxide with the anatase structure on the photoanode surface. Thus, samples containing 0.5 to 20 wt % of Bi are obtained. It is shown that the doping of titanium dioxide by bismuth ions results in a shift of light absorption to the visible region of electromagnetic radiation spectrum. The absorption level depends on the concentration of bismuth and reaches its maximum for samples containing 0.5 and 1.0 wt % of Bi. It is suggested on the basis of the data of X-ray phase analysis that an increase in the content of bismuth to 20 wt % leads to destruction of crystalline regions and amorphization of bismuth oxide and titanium oxide. The obtained coatings are studied as catalysts of photoelectrocatalytic oxidation of formic acid under illumination by monochromatic and visible light. It is found that the highest catalytic effect is observed on samples containing 1.0 wt % of bismuth. The forbidden gap width is estimated on the basis of absorption of monochromatic (464 nm) light, and it is shown that photoelectrocatalytic oxidation of formic acid in the visible spectral range accompanied by formiate ion adsorption on the illuminated photoanode surface is probably due to a decrease in the forbidden gap width in doped titanium dioxide to 2.7 eV.







Similar content being viewed by others
REFERENCES
Fujishima, A. and Honda, K., Nature, 1972, vol. 238, p. 37.
Ni, M., Leung, M.K., and Leung, D.Y.C., Int. J. Hydrogen Energy, 2007, vol. 32, no. 13, p. 2305.
Ma, Y., Wang, X., Jia, Y., et al., Chem. Rev., 2014, vol. 114, no. 19, p. 9987.
Li, X., Yu, J., Low, J., et al., J. Mater. Chem. A, 2015, vol. 3, no. 6, p. 2485.
Chen, X., Shen, S., Guo, L., et al., Chem. Rev., 2010, vol. 110, no. 11, p. 6503.
Warren, E.L., McKone, J.R., Boettcher, S.W., et al., Chem. Rev., 2010, vol. 110, p. 6446.
Kudo, A. and Miseki, Y., Chem. Soc. Rev., 2009, vol. 38, no. 1, p. 253.
Maeda, K., J. Photochem. Photobiol., C, 2011, vol. 12, no. 4, p. 237.
Han, F., Kambala, V.S., Srinivasan, M., et al., Appl. Catal., A, 2009, vol. 359, nos. 1–2, p. 25.
Antopoulou, M., Evgenidou, E., Lambropoulou, D., et al., Water Res., 2014, vol. 53, p. 215.
Pelaez, M., Nolan, N.T., Pillai, S.C., et al., Appl. Catal., B, 2012, vol. 125, p. 331.
Hoffmann, M.R., Martin, S.T., Choi, W., et al., Chem. Rev., 1995, vol. 95, p. 69.
Akpan, U.G. and Hameed, B.H., J. Hazard. Mater., 2009, vol. 170, nos. 2–3, p. 520.
Fujishima, A., Rao, T.N., and Tryk, D.A., J. Photochem. Photobiol., C, 2000, vol. 1, no. 1, p. 1.
Chen, C., Ma, W., and Zhao, J., Chem. Soc. Rev., 2010, vol. 39, no. 11, p. 4206.
Park, H., Park, Y., Kim, W., et al., J. Photochem. Photobiol., C, 2013, vol. 15, no. 1, p. 1.
Shiraishi, Y. and Hirai, T., J. Photochem. Photobiol., C, 2008, vol. 9, no. 4, p. 157.
Lincebigler, A.L., Lu, G., and Yates, J.T., Chem. Rev., 1995, vol. 95, no. 3, p. 735.
Xu, Q., Yu, J., Zhang, J., et al., Chem. Commun., 2015, vol. 51, no. 37, p. 7950.
Yu, J., Low, J., Xiao, W., et al., J. Am. Chem. Soc., 2014, vol. 136, no. 25, p. 8839.
Li, X., Wen, J.Q., Low, J.X., et al., Sci. China Chem., 2014, vol. 57, p. 70.
Fu, J., Cao, S., Yu, J., et al., Dalton Trans., 2014, vol. 43, no. 24, p. 9158.
Li, X., Liu, H.L., Luo, D.L., et al., Chem. Eng. J., 2012, vol. 180, p. 151.
Dhakshinamoorthy, A., Navalon, S., Corma, A., et al., Energy Environ. Sci., 2012, vol. 5, no. 11, p. 9217.
Zhang, Q.H., Han, W.D., Hong, Y.J., et al., Catal. Today, 2009, vol. 148, nos. 3–4, p. 335.
Hemminger, J.C., Carr, R., and Somorjai, G.A., Chem. Phys. Lett., 1978, vol. 57, no. 1, p. 100.
Inoue, T., Fujishima, A., Konish, S., and Honda, K., Nature, 1979, vol. 227, p. 637.
Hirano, K. and Bard, A.J., J. Electrochem. Soc., 1980, vol. 127, no. 5, p. 1056.
Reiche, H. and Bard, A.J., J. Am. Chem. Soc., 1979, vol. 101, no. 11, p. 3127.
Kanno, T., Oguchi, T., Sakuragi, H., et al., Tetrahedron Lett., 1980, vol. 21, no. 5, p. 467.
Taniguchi, I., Nakashima, K., Yamaguchi, H., and Yasukouchi, K., J. Electroanal. Chem., 1982, vol. 134, p. 191.
Pleskov, Yu.V., Elektrokhimiya, 1981, vol. 17, p. 3.
Grinberg, V.A., Dzhavrishvili, T.V., Vasil’ev, Yu.B., et al., Elektrokhimiya, 1984, vol. 20, no. 1, p. 121.
Gurevich, Yu.Ya. and Pleskov, Yu.V., Itogi Nauki Tekh., Ser.: Elektrokhim., 1982, vol. 18, p. 3.
Mills, A. and Le Hunte, S., J. Photochem. Photobiol., A, 1997, vol. 108, no. 1, p. 1.
Mills, A. and Lee, S.K., J. Photochem. Photobiol., A, 2002, vol. 152, nos. 1–3, p. 233.
Anpo, M. and Takeuchi, M., J. Catal., 2003, vol. 216, p. 505.
Antoniadou, M. and Lianos, P., J. Nanosci. Nanotechnol., 2010, vol. 10, no. 9, p. 6240.
Li, L., Zhang, S., Li, G., and Zhao, H., Anal. Chem. Acta, 2012, vol. 754, p. 47.
Grinberg, V.A., Emets, V.V., Modestov, A.D., et al., Russ. J. Electrochem., 2017, vol. 53, no. 2, p. 217.
Wen, J., Li, X., Liu, W., et al., Chin. J. Catal., 2015, vol. 36, no. 12, p. 2049.
Su, R., Bechstein, R., Kibsgaard, J., et al., J. Mater. Chem., 2012, vol. 22, no. 45, p. 23 755.
Tu, Y.F., Huang, S.Y., Sang, J.P., et al., Mater. Res. Bull., 2010, vol. 45, no. 2, p. 224.
Grinberg, V.A., Emets, V.V, Maiorova, N.A, et al., Prot. Met. Phys. Chem. Surf., 2018, vol. 54, no. 1, p. 51.
Baifu Xin, Liqiang Jing, Zhiyu Ren, et al., J. Phys. Chem. B, 2005, vol. 109, p. 2805.
Sajjad, S., Leghari, S.A.K., Chen, F., et al., Chem. - Eur. J., 2010, vol. 16, no. 46, p. 13 795.
Andronic, L., Enesca, A., Vladuta, C., and Duta, A., Chem. Eng. J., 2009, vol. 152, p. 64.
Hajjaji, A., Atyaoui, A., Trabelsi, K., et al., Am. J. Anal. Chem., 2014, vol. 5, no. 8, p. 473.
Hoang, S., Berglund, S.P., Hahn, N.T., et al., J. Am. Chem. Soc., 2012, vol. 134, p. 3659.
Hoang, S., Guo, S.W., Hahn, N.T., et al., Nano Lett., 2011, vol. 12, p. 26.
Wang, G.M., Wang, H.Y., Ling, Y.C., et al., Nano Lett., 2011, vol. 11, p. 3026.
Maksimov, Yu.V., Suzdalev, I.P., Tsodikov, M.V., et al., J. Mol. Catal. A: Chem., 1996, vol. 105, no. 3, p. 167.
Kriventsov, V.V., Kochubey, D.I., Tsodikov, M.V., et al., Nucl. Instrum. Methods Phys. Res., Sect. A, 2001, vol. 407, nos. 1–2, p. 331.
Jiang, D., Zhao, H., Zhang, S., et al., J. Phys. Chem. B, 2003, vol. 107, no. 46, p. 12 774.
Zhang, H., Zhao, H., Zhang, S., et al., ChemPhysChem, 2008, vol. 9, p. 117.
Kim, D.H. and Anderson, M.A., J. Photochem. Photobiol., A, 1996, vol. 94, nos. 2–3, p. 221.
Lana-Villarreal, T., Goґmez, R., Neumann-Spallart, M., et al., J. Phys. Chem. B, 2004, vol. 108, p. 15 172.
Lana-Villarreal, T., Peґrez, J.M., and Goґmez, R., C. R. Chim., 2006, vol. 9, p. 806.
Gong, X.-Q., Selloni, A., and Vittadini, A., J. Phys. Chem. B, 2006, vol. 110, p. 2804.
Montoya, J.F., Peral, J., and Salvador, P., J. Phys. Chem. C, 2014, vol. 118, no. 26, p. 14 266.
Montoya, J.F., Atitar, M.F., and Bahnemann, D.W., J. Phys. Chem. C, 2014, vol. 118, p. 14 276.
Kim, D.H. and Anderson, M.A., Environ. Sci. Technol., 1994, vol. 28, no. 3, p. 479.
Mitoraj, D., Beranek, R., and Kisch, H., Photochem. Photobiol. Sci., 2010, vol. 9, no. 1, p. 31.
ACKNOWLEDGMENTS
Absorption spectra of nanosize films of titanium dioxide doped by bismuth were obtained using the equipment of Center for Collective Use of Physical Research Methods of the Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences.
Funding
The work was supported by the Program of Fundamental Research of Presidium of Russian Academy of Sciences 1.8P “Fundamental Aspects of Chemistry of Carbon Energetics” and the State task for the Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences, theme no. 47.23.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by M. Ehrenburg
Rights and permissions
About this article
Cite this article
Grinberg, V.A., Emets, V.V., Mayorova, N.A. et al. Photoelectrocatalytic Oxidation of Formic Acid in the Visible Spectral Region on Films of Nanocrystalline Titanium Oxide Doped by Bismuth. Prot Met Phys Chem Surf 55, 637–645 (2019). https://doi.org/10.1134/S2070205119040051
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205119040051