Abstract
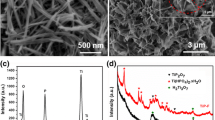
We report a facile and reproducible method to synthesize advanced, homogenized, hybrid, nanoflower of thorium oxide and thorium oxalate hydrate material via a novel, green, microwave irradiated chemical process. The Nanoflowers can be successfully synthesized using thorium nitrate penta hydrate as the metal source along with two different capping agents, cetyltrimethyl ammonium bromide and 4-amino-1H-pyrimidine-2-one respectively in the ubiquity of microwave irradiation having power source 230V at the temperature of 45°C for 15 minutes to get the desired product. The synthesized material was characterized by various complementary techniques namely XRD, FTIR, PL, TGA/DSC curve, SEM and EDX. The 3D nanoflowers structure, so formed, resembles a natural Peony flower. The applications of synthesized material lies in the area of making thorium metal, densified thorium oxide, carbide and nitride, anhydrous thorium complexes and thorium boron silicates glasses.





Similar content being viewed by others
REFERENCES
Lee, S.W., Cheon, S.A., Kim, M.I., and Park, T.J., J. Nanobiotechnol., 2015, vol. 13, p. 5.
Ge, J., Lei, J., and Zare, R.N., Nat. Nanotechnol., 2012, vol. 7, p. 428.
Kharisov, B.I., Recent Pat. Nanotechnol., 2008, vol. 2, p. 190.
Lim, B., Jiang, M., Camargo, P.H., Cho, E.C., Tao, J., and Lu, X., Science, 2009, vol. 324, p. 130.
Ning, J., Dai, Q., Jiang, T., Men, K., Liu, D., Xiao, N., Chenyuan, Li, Li, D., Liu, B., Zou, B., Zou, G., and Yu, W.W., Langmuir, 2009, vol. 25, p. 1818.
Ge, J., Lei, J., and Zare, R.N., Nat. Nanotechnol., 2012, vol. 7, p. 42.
Sun, Z., Kim, J.M., Zhao, Y., Bijarbooneh, F., Malgras, V., Lee, Y., Kang, Y.M., and Dou, S.X., J. Am. Chem. Soc., 2011, vol. 193, p. 133.
Mohanty, A., Garg, N., Jin, R., Angew. Chem., Int. Ed., 2010, vol. 49, p. 4962.
Tang, Y., Rui, X., Zhang, Y., Lim, T.M., Dong, Z., Hng, H.H., Chen, X., Yan, Q., and Chen, Z., J. Mater. Chem. A, 2013, vol. 1, p. 82
http://en.wikipedia.org/wiki/India%27s_three-stage_nuclear_power_programme.
Ananthasivan, K., Balakrishnan, S., Anthonysamy, S., Divakar, R., Mohandas, E., and Ganesan, V., J. Nucl. Mater., 2013, vol. 434, p. 223.
Curran, G., Sevestre, Y., Rattray, W., Allen, P., and Czerwinski, K.R., J. Nucl. Mater., 2003, vol. 41, p. 323.
Niranjan, R.S., Londhe, M.S., Mandale, A.B., et al., Sens. Actuators, B, 2002, vol. 87, 406.
Ho, S.W., J. Catal., 1998, vol. 175, p. 139.
Ge, F.Z., Ye, Z.Z., Wang, F.Z., Zhang, Y.P., and Ding, B.J., Mater. Lett., 2003, vol. 57, p. 2776.
Zhang, H., Chen, X.Y., Yang, Z.M., and Ding, B.J., Mater. Lett., 1999, vol. 38, p. 401.
Moeini, M., Malekzadeh, A., Ahmadi, S.J., and Hosseinpour, M.D.F., Mater. Lett., 2012, vol. 81, p. 99.
Wang, L., Zhao, R., Wang, X.W., Mei, L., Yuan, L., Wang, S., Chai, Z., and Shi, W., CrystEngComm, 2014, vol. 16, p. 10469.
Batuk, O.N., Szabo, D.V., Denecke, M.A., Vitova, T., and Kalmykov, S.V., Radiochim. Acta, 2013, vol. 101, no. 4, p. 233.
Verma, S. and Amritphale, S.S., J. Radioanal. Nucl. Chem., 2016, vol. 307, p. 669.
Bajia, S., Sharma, R., and Bajia, B., E-J. Chem., 2009, vol. 6, p. 120.
Verma, S., Amritphale, S.S., and Das, S., J. Chem. Res., 2016, vol. 40, p. 323.
Zhang, P., Yin, S., and Satp, T., Appl. Catal., B, 2009, vol. 89, p. 118.
Katsuyuki, O., Jin, O., and Yoshiharu, T., US Patent 4364859A, 1982.
Balakrishna, P., Nat. Sci., 2015, vol. 7, pp. 10–17.
Simpson, M.P. and Morrell, M.S., Electron. Power, 1982, vol. 28, pp. 612–613.
Sharma, P., Dutta, R., Liu, K., Aninash, R.P., and Pandey, A.C., Mater. Lett., 2010, vol. 64, p. 1183.
Chen, W., Mai, L., Qi, Y., and Dai, Y., J. Phys. Chem. Solids, 2006, vol. 67, p. 896.
Ali, M.E. and Lamprecht, A., J. I. J. Pham., 2003, vol. 45, p. 6135.
Powder Diffraction File, Alphabetical Index Inorganic Phases, Swarthmore, PA: JCPDS International Centre for Diffraction Data, 1984.
Hussein, G.A.M. and Ismail, H.M., Colloids Surf., A, 1995, vol. 99, p. 129.
Nakamoto, K., Infrared and Raman Spectra of Inorganic and Coordination Compounds, Hoboken, NJ: John Wiley and Sons, 2009, pp. 766–773.
Socrates, G., Infrared and Raman Characteristic Group Frequencies: Table and Charts, New York: John Wiley and Sons, 2004, p. 287.
Lin, Z.W., Kuang, Q., Lian, W., Jiang, Z.Y., Xie, Z.X., Huang, R.B., and Zheng, L.S., J. Phys. Chem. B, 2006, vol. 110, p. 2307.
Beckett, R. and Winfield, M.E., Aust. J. Sci. Res., 1951, vol. 4, p. 644.
Aybers, M.T., J. Nucl. Mater., 1998, vol. 252, p. 28.
Walther, C., Rothe, J., Schimmelpfennig, B., and Fuss, M., Dalton Trans., 2012, vol. 41, p. 10941.
Moeini, M., Malekzadeh, A., Ahmadi, S.J., and Hosseinpour, M., Mater. Lett., 2012, vol. 81, p. 99.
Tiwari, R.N. and Sinha, D.N., J. Indian Chem., 1980, vol. 14, p. 25.
Jonke, A.A., Petkus, E.J., Loeding, J.W., and Lawroski, S., Nucl. Sci. Eng., 1957, vol. 2, p. 303.
Zagorski, Z.P. and Gluszewski, W., Nukleonika, 2010, vol. 55, p. 407.
Jaqueline, Y. and Thibault, L.K., US Patent 8431689B2, 2010.
ACKNOWLEDGMENTS
Authors are grateful to Director CSIR-AMPRI Bhopal for providing necessary institutional facilities and encouragement. Thanks are also due to Dr. D.P. Mondal, Mr. Mohd. Shafique and Mr. Deepak Kashyap of CSIR- AMPRI for analysis of samples on SEM, EDX and providing data of thermal analysis of samples. Dr Neelesh Jain, SIRT, Bhopal and MANIT, Bhopal for providing facilities for IR and PL visible spectra.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Sarika Verma, Mishra, D., Sanghi, S.K. et al. An Instant, Green, Microwave Irradiated Process for the Preparation of Advanced, Hybrid, Nanoflower of Thorium Oxide and Thorium Oxalate Hydrate Useful for Broad Application Spectrum. Prot Met Phys Chem Surf 55, 65–71 (2019). https://doi.org/10.1134/S2070205119010246
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205119010246