Abstract
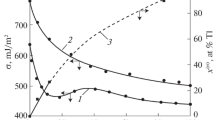
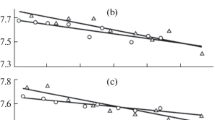
A molecular theory based on the lattice gas model is employed to describe the surface tension of the vapor–liquid interfaces of one- and two-component metal melts. The surface tension of the melts are calculated in the quasi-chemical approximation of taking into account intermolecular interactions of the nearest neighbors. Parameters of the model are found from the experimental data for the bulk surface tension of the melts, which enables the calculation of the surface tensions of vapor–liquid interfaces of one- and two-component droplets with different sizes as a function of their radii. Estimates for the minimum size of small droplets of melts having the properties of a homogeneous phase inside them, which correspond to their thermodynamic stability, are obtained.
Similar content being viewed by others
References
Adamson, A.W., Physical Chemistry of Surfaces, New York, London, Sydney, Toronto: John Wiley and Sons, 1975.
Khokonov, Kh.B., Poverkhnostnye yavleniya v rasplavakh i voznikayushchikh iz nikh tverdykh fazakh (Surface Phenomena in Melts and Solid Phases which Appear in Melts), Chisinau: Shtiintsa, 1974.
Nizhenko, N.I. and Floka, L.I., Poverkhnostnoe natyazhenie zhidkikh metallov i splavov (Surface Tension in Liquid Metals and Alloys), Moscow: Metallurgiya, 1981.
Jaycock, M.J. and Parfitt, G.D., Chemistry of Interfaces, New York: John Wiley and Sons, 1981.
Semenchenko, V.K., Poverkhnostnye yavleniya v metallakh i splavakh (Surface Phenomena in Metals and Alloys), Moscow: Gostekhizdat, 1957.
Volmer, M., Kinetik der Phasenbildung, Drezden, Leipzig: Theodor Steinkopff, 1939.
Frenkel’, Ya.I., Kineticheskaya teoriya zhidkostei (Kinetic Theory of Liquids), Moscow, Leningrad: USSR Acad. Sci., 1945.
Rusanov, A.I., Fazovye ravnovesiya i poverkhnostnye yavleniya (Phase Equilibria and Surface Phenomena), Leningrad: Khimiya, 1967.
Buff, F.P. and Kirkwood, J.G., J. Chem. Phys., 1950, vol. 18, p.991.
Abraham, F.F., Homogeneous Nucleation Theory. The Pretransition Theory of Vapor Condensation, New York: Academic Press, 1974.
Lushnikov, A.A. and Sutugin, A.G., Usp. Khim., 1976, vol. 45, p.385.
Lushnikov, A.A., Dokl. Akad. Nauk SSSR, 1977, vol. 234, p.97.
Ono, S. and Kondo, S., Molecular Theory of Surface Tension in Liquids, Berlin, Gottinhen, Heidelberg: Springer, 1960.
Rowlinson, J.S. and Widom, B., Molecular Theory of Capillarity, Oxford: Clarendon Press, 1982.
Croxton, C.A., Liquid State Physics–Statistical Mechanical Introduction, Cambridge: Cambridge Univ. Press, 1974.
Tovbin, Yu.K., Russ. J. Phys. Chem. A, 2010, vol. 84, no. 2, p.180.
Tovbin, Yu.K., Zaitseva, E.S., and Rabinovich, A.B., Russ. J. Phys. Chem. A, 2017, vol. 91, no. 10, p. 1957.
Zaitseva, E.S. and Tovbin, Yu.K., Russ. J. Phys. Chem. A, 2017, vol. 91, no. 11, p. 2208.
Tovbin, Yu.K. and Zaitseva, E.S., High Temp., 2018, vol. 56 (in press).
Tovbin, Yu.K., Theory of Physical Chemistry Processes at a Gas–Solid Surface Processes, Boca Raton, FL: CRC Press, 1991.
Tovbin, Yu.K., Molecular Theory of Adsorption in Porous Bodies, Boca Raton, FL: CRC Press, 2018.
Tovbin, Yu.K., Kolloidn. Zh., 1983, vol. 45, no. 4, p.707.
Iwamatsu, M., J. Phys.: Condens. Matter, 1994, vol. 6, p.173.
Baidakov, V.G. and Boltachev, G.Sh., Zh. Fiz. Khim., 1995, vol. 69, p.515.
Oxtoby, D.W. and Evans, R., J. Chem. Phys., 1988, vol. 89, p. 7521.
Bykov, T.V. and Shchekin, A.K., Inorg. Mater., 1999, vol. 35, no. 6, p.641.
Bykov, T.V. and Shchekin, A.K., Colloid J., 1999, vol. 61, no. 2, p.144.
Moody, M.P. and Attard, P., J. Chem. Phys., 2002, vol. 117, p. 6705.
He, S. and Attard, P., Chem. Phys., 2005, vol. 7, p. 2928.
Thompson, S.M., Gubbins, K.E., Walton, J.P.R., et al., J. Chem. Phys., 1984, vol. 81, p.530.
Zhukhovitskii, D.I., Colloid J., 2003, vol. 65, no. 4, p.440.
Gibbs, J.W., Elementary Principles in Statistical Mechanics, New York: Dover Publ., 1960.
Hirschfelder, J.O., Curtiss, C.F., and Bird, R.B., Molecular Theory of Gases and Liquids, New York: John Wiley and Sons, 1954.
Prigogine, I.P., The Molecular Theory of Solutions, Amsterdam, New York, NY: Interscience Publ., 1957.
Bird, R.B., Stewart, W.E., and Lightfoot, E.N., Transport Phenomena, New York: John Wiley and Sons, 1960.
Reid, R., Prausnitz, J., and Sherwood, T., The Properties of Gases and Liquids, New York: McGraw-Hill, 1977.
Gupta, R.P., Phys. Rev. B, 1981, vol. 23, p. 6265.
Cleri, F. and Rosato, V., Phys. Rev. B, 1993, vol. 1993 V, p.22.
Paz-Borbón, L.O., Computational Studies of Transition Metal Nanoalloys, Berlin, Heidelberg: Springer, 2011.
Solov’yov, I.A., Korol, A.V., and Solov’yov, A.V., Multiscale Modeling of Complex Molecular Structure and Dynamics with MBN Explorer, Springer Int., 2017.
Tablitsy fizicheskikh velichin. Spravochnik (Tables of Physical Quantities. Handbook), Kikoin, I.K., Ed., Moscow: Atomizdat, 1976.
Basin, A.S., Butlerov. Soobshch., 2002, no. 83, p.83.
Bokshtein, B.S., Bokshtein, S.Z., and Zhukhovitskii, A.A., Termodinamika i kinetika diffuzii v tverdykh telakh (Thermodynamics and Kinetics of Diffusion in Solids), Moscow: Metallurgiya, 1974.
Bokshtein, B.S., Diffuzii v metallakh (Diffusions in Metals), Moscow: Metallurgiya, 1978.
Mehrer, H., Diffusion in Solids, vol. 155 of Springer Series in Solid-State Sciences, Berlin, Heidelberg: Springer, 2007.
Drits, M.E., Svoistva elementov. Spravochnik (Properties of Elements. Handbook), Moscow: Metallurgiya, 1985.
Han, X.J. and Wei, B., Philos. Mag., 2003, vol. 83, no. 13, p. 1511.
Xiao Feng, et al., Trans. Nonferrous Met. Soc. China, 2008, no. 18, p. 1184.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.S. Zaitseva, Yu.K. Tovbin, 2018, published in Fizikokhimiya Poverkhnosti i Zashchita Materialov, 2018, Vol. 54, No. 5, pp. 415–419.
Rights and permissions
About this article
Cite this article
Zaitseva, E.S., Tovbin, Y.K. Size Characteristics of the Surface Tension of One- and Two-Component Metal Melts. Prot Met Phys Chem Surf 54, 749–753 (2018). https://doi.org/10.1134/S2070205118050246
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205118050246