Abstract
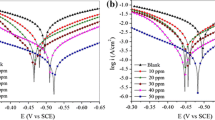
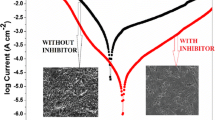
Two novel aza-pseudopeptides such as 2-(1-(3-methyl-5,6-dihydropyridazin-1(2H)-yl)-1-oxopropan-2-yl)isoindoline-1,3-dione (E10) and 2-(2-(5-(4-(dimethylamino) phenyl)-4,5-dihydro-1H-pyrazol- 1-yl)-2-oxoethyl) isoindoline-1,3-dione (E12) were synthesized, characterized by FTIR, 1H NMR, 13C NMR, and tested as corrosion inhibitors for mild steel in 1.0 M HCl. The inhibition effect of the prepared compounds was studied by electrochemical impedance spectroscopy, Tafel polarization and quantum chemical calculations. The experimental results suggest that these compounds are efficient corrosion inhibitors and the inhibition efficiencies increase with increasing their concentrations. The efficiencies obtained from EIS measurements were in good agreement with those obtained from the polarization measurements. Polarization curves revealed that the prepared compounds act as mixed type inhibitors. The interaction between inhibitor and surface of steel obey Langmuir isotherm. The density functional theory was employed for theoretical calculations, and the obtained results were found to be consistent with the experimental findings.
Similar content being viewed by others
Change history
15 March 2018
The name of the second author should read A. M. Elhorri.
15 March 2018
The name of the second author should read A. M. Elhorri.
References
Sastri, V.S., Corrosion Inhibitors, New York: John Wiley & Sons, 1998.
Zarrouk, A., Hammouti, B., Zarrok, H., et al, Int. J. Electrochem. Sci., 2012, vol. 6, p. 89.
Ghazoui, A., Saddik, R., Benchat, N., et al, Int. J. Electrochem. Sci., 2012, vol. 7, p. 7080.
Quraishi, M.A., Sardar, R., and Jamal, D, Mater. Chem. Phys., 2001, vol. 71, p. 309.
Eddy, N.O., Ameh, P.O., Gwarzo, M.Y., et al, Port. Electrochim. Acta, 2013, vol. 31, p. 79.
Joseph, B., Prajila, M., and Joseph, A., J. Dispersion Sci. Technol., 2012, vol. 33, p. 739.
Zarrok, H., Oudda, H, El Midaoui, A., et al., Res. Chem. Intermed., 2012, vol. 38, p. 2051.
Varalakshm, C. and Appa Rao, B.V, Anti-Corros. Methods Mater., 2001, vol. 48, p. 171.
Bentiss, F., Traisnel, M., and Lagrenee, M, Corros. Sci., 2002, vol. 42, p. 127.
Tayebi, H., Bourazmi, H., Himmi, B., et al, Pharm. Lett., 2014, vol. 6, p. 20.
Tayebi, H., Bourazmi, H., Himmi, B., et al, Pharma Chem., 2014, vol. 6, p. 220.
Kabanda, M.M., Murulana, L.C., Ozcan, M., et al, Int. J. Electrochem. Sci., 2012, vol. 7, p. 5035.
Ahamad, I., Prasad, R., and Quraishi, M.A, Mater. Chem. Phys., 2010, vol. 124, p. 1155.
Musa, A.Y., Jalgham, R.T.T., and Mohamad, A.B, Corros. Sci., 2012, vol. 56, p. 176.
Klausner, Y. and Bodansky, M, Synthesis, 1974, vol. 8, p. 549.
Paul, R. and Anderson, W, J. Am. Chem. Soc., 1960, vol. 824, p. 596.
Chu, W., Tu, Z., McElveen, E., et al, Bioorg. Med. Chem., 2005, vol. 13, p. 77.
Chicharro, R., Castro, S., Reino, J., and Aran, V, Eur. J. Org. Chem., 2003, vol. 12, p. 2314.
Pizey, J.S, Synthetic Reagents, New York: John Wiley & Sons, 1974, vol. 1, p. 321.
Pearson, A.J. and Roush, W.R, Handbook of Reagents for Organic Synthesis: Activating Agents and Protecting Groups, New York: John Wiley & Sons, 1999, p. 370
Chu, W., Tu, Z., McElveen, E., et al., Bioorg. Med. Chem., 2005, vol. 13, p. 77.
Sänchez-Sancho, F., Mann, E., and Herradön, B., SYNLETT, 2000, vol. 4, p. 509.
Medjahed, W., TabetZatla, A, Kajima Mulengi, J., et al., Tetrahedron Lett., 2004, vol. 45, p. 1211.
Becke, A.D, J. Chem. Phys., 1992, vol. 96, p. 9489.
Becke, A.D, J. Chem. Phys., 1993, vol. 98, p. 1372.
Lee, C., Yang, W., and Parr, R.G, Phys. Rev. B, 1988, vol. 37, p. 785.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., et al., Gaussian 03, Revision B.01, Pittsburgh, PA: Gaussian, 2003.
Ferreira, E.S., Giancomelli, C., Giacomelli, F.C., and Spinelli, A, Mater. Chem. Phys., 2004, vol. 83, p. 129.
Quraishi, M.A., Khan, M.A.W., Ajmal, M., et al, J. Appl. Electrochem., 1996, vol. 26, p. 1253.
Ali, S.A., El-Shareef, A.M., Al-Ghandi, R.F., and Saeed, M.T, Corros. Sci., 2005, vol. 47, p. 2659.
Deyab, M.A, Abd El-Rehim, S.S., and Keera, S.T., Colloids Surf., A, 2009, vol. 348, p. 170.
Haruyama, S., Tsuru, T., and Gijutsu, B, J. Jpn. Soc. Corros. Eng., 1978, vol. 27, p. 573.
Elkadi, L., Mernari, B., Traisnel, M., et al, Corros. Sci., 2000, vol. 42, p. 703.
Saliyan, V.R. and Adhikari, A.V, Corros. Sci., 2008, vol. 50, p. 55.
Atta, A.M., El-Azabawy, O.E., Ismail, H.S., and Hegazy, M.A, Corros. Sci., 2011, vol. 53, p. 1680.
Khaled, K.F., El-Mghraby, A., Ibrahim, O.B., et al, J. Mater. Environ. Sci., 2010, vol. 1, p. 139.
Yuce, A.O., Mert, B.D., Kardas, G., and Yazıcı, B, Corros. Sci., 2014, vol. 83, p. 310.
Al-Sabagh, A.M., Migahed, M.A., and Awad, H.S, Corros. Sci., 2006, vol. 48, p. 813.
Ozkır, D., Kayakırılmaz, K., Bayol, E., et al, Corros. Sci., 2012, vol. 56, p. 143.
Godec, R.F, Electrochim. Acta, 2009, vol. 54, p. 2171.
Ma, H., Chen, S., Liu, Z., and Sun, Y, J. Mol. Struct.: THEOCHEM, 2006, vol. 77, p. 419.
Yadav, D.K., Maiti, B., and Quraishi, M.A, Corros. Sci., 2010, vol. 52, p. 3586.
Fu, J.J., Zang, H., Wang, Y., et al, Ind. Eng. Chem. Res., 2012, vol. 51, p. 6377.
Gece, G, Corros. Sci., 2008, vol. 50, p. 2981.
Obot, I.B. and Obi-Egbed, N.O, Corros. Sci., 2011, vol. 53, p. 263.
Zhao, P., Liang, Q., and Li, Y, Appl. Surf. Sci., 2005, vol. 252, p. 1596.
Khalil, N, Electrochim. Acta, 2003, vol. 48, p. 2635.
Fleming, I., Frontier Orbitals and Organic Chemical Reactions, New York: John Wiley & Sons, 1976.
Khaled, K.F, Mater. Chem. Phys., 2008, vol. 112, p. 104.
Khaled, K.F, Electrochim. Acta, 2008, vol. 53, p. 3484.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Chadli, R., ELherri, A., Elmsellem, H. et al. Synthesis of aza-pseudopeptides and the evaluation of their inhibiting efficacy of mild steel corrosion in 1.0 M HCl. Prot Met Phys Chem Surf 53, 928–936 (2017). https://doi.org/10.1134/S2070205117050033
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205117050033