Abstract
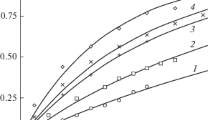
Transport processes that take place during the anodic dissolution of a metal accompanied by the formation of negatively charged intermediate particles are theoretically considered. Distributions of the component concentrations and electric potential in the diffusion layer during the quasi-equilibrium decomposition of intermediate particles are calculated. Analysis predicts the existence of the admissible concentration limit of anions depending on the stability constant of intermediate particles. The effect of the stability constant on the potential distribution within the diffusion layer and on the shape of voltammetric curves of the process is found to be complex.
Similar content being viewed by others
References
Ait’yan, S.Kh., Davydov, A.D., and Kabanov, B.N., Elektrokhimiya, 1972, vol. 8, no. 4, p. 620.
Ait’yan, S.Kh., Davydov, A.D., and Kabanov, B.N., Elektrokhimiya, 1972, vol. 8, no. 9, p. 1391.
Krylov, V.S., Davydov, A.D., and Malienko, V.N., Elektrokhimiya, 1972, vol. 8, no. 10, p. 1461.
Noskov, A.V. and Lilin, S.A., Zashch. Met., 2004, vol. 40, no. 2, p. 137.
Noskov, A.V. and Lilin, S.A., Zashch. Met., 2007, vol. 43, no. 2, p. 117.
Noskov, A.V. and Lilin, S.A., Fizikokhim. Poverkhn. Zashch. Mater., 2009, vol. 45, no. 1.
Dvorak, J., Koryta, J., and Bohackova, V., Elektrochemie (Electrochemistry), Praha: Academia, 1975.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.V. Noskov, S.A. Lilin, 2009, published in Fizikokhimiya Poverkhnosti i Zashchita Materialov, 2009, Vol. 45, No. 6, pp. 664–668.
Rights and permissions
About this article
Cite this article
Noskov, A.V., Lilin, S.A. Anodic dissolution of a metal accompanied by the formation of unstable anionic complexes. Prot Met Phys Chem Surf 45, 746–751 (2009). https://doi.org/10.1134/S2070205109060185
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205109060185