Abstract
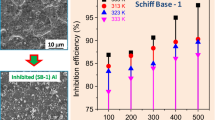
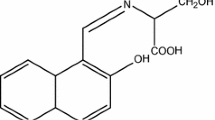
The effect of Schiff’s bases alone and Schiff’s bases with additive Na2SO4 on the corrosion of aluminium in H2SO4 have been investigated by using weight loss method. The present study revealed that aluminium in H2SO4 has been more efficiently inhibited by Schiff’s bases in the presence of additive Na2SO4 than Schiff’s bases alone due to the synergistic effect between Schiff’s bases and Na2SO4. Inhibition efficiency was found maximum upto 95.02% for aluminium in H2SO4 by Schiff’s bases in presence of additive NaNa2SO4. The adsorption of inhibitor accords with the Langmuir adsorption isotherm. Results obtained in both the cases indicate the dependence of inhibition efficiencies on the concentration of Schiff’s bases, additive Na2SO4 and also on the concentration of H2SO4 solution. The results show the increasing trends of inhibition efficiency with the concentration.
Similar content being viewed by others
References
Uligh, H.H., Corrosion and Corrosion Control an Introduction to Corrosion Science and Engineering, N.Y.: Wiley & Sons, 1971.
Bastidas, J.M. and Otero, E., Mater. Corros., 1996, vol. 47, p. 333.
Nathan, C.C. and Perugini, J.J., Materials Performance, 1974, vol. 13, p. 29.
Porter, F.C., Metalurgia, 1962, vol. 65, p. 65.
Aziz, P.M. and Godard, H.P., Corrosion, 1959, vol. 15, p. 529.
Ebenso, E.E., Okafor, P.C., and Eppe, U.J., Anticorr. Meth. Mat., 2003, vol. 50, no. 6, p. 414.
Blanc, C., Gastaud, S., and Mankowski, G., J. Electrochem. Soc., 150(B) 2003, vol. 150, p. 396.
Mosaleva, A., Poznyok, A., Mozaleva, I., et al., Electrochem. Comm., 2001, vol. 3, p. 299.
Klassen, R.D., Hyatt, C.V., Roberge, P.R., et al., J. Canadian Metallurgical, 2002, vol. 41, no. 1, p. 121.
Bazzi, L., Salghi, R., Zine, E., et al., Can. J. Chem., 2002, vol. 80, no. 1, p. 106.
Quafsaoui, W., Blanc, C.H., Bebere, N., et al., J. Appl. Electrochem., 2000, vol. 30, p. 959.
Putilova, I.N., Balizin, S.A., and Baranmik, V.P., Metallic Corrosion Inhibitors, 1960.
Upadhyay, R.K., Anthony, S., and Mathur, S.P., Russ. J. Electrochem., 2007, vol. 43, p. 238.
Sethi, T., Chaturvedi, A., Upadhyay, R.K., et al., Boletin De La Sociedad Chilena De quimica, 2007, vol. 52, no. 3, p. 1136.
Sethi, T., Chaturvedi, A., Upadhyay, R.K., et al., Polish J. Chem., 2008, vol. 82, p. 591.
Anderson, E.E., Duca, C.J., and Scudi, J.V., J. Am. Chem. Soc., 1951, vol. 73, p. 4967.
Dodoff, N.I., Ozdemir, V., Karacan, N., et al., Naturfarch Z., 1993, vol. 548, p. 1553.
Erk, B., Dilmac, A., Baran, Y., et al., Synth. React. Inorg Met. Org. Chem., 2000, vol. 10, p. 30.
Jones, D.A., Principle and Prevention of Corrosion (2nd Ed.), London: Prentice Hall International Limited, 1996.
Suetaka, W., Bull. Chem. Soc., 1964, vol. 37, p. 112.
Hoar, T.P. and Holliday, R.D., J. App. Chem., 1953, vol. 3, p. 582.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Sethi, T., Chaturvedi, A., Upadhyaya, R.K. et al. Synergistic inhibition between Schiff’s bases and sulfate ion on corrosion of aluminium in sulfuric acid. Prot Met Phys Chem Surf 45, 466–471 (2009). https://doi.org/10.1134/S2070205109040169
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205109040169