Abstract
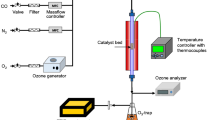
The deactivation of the commercial Al-Pd catalyst promoted with silver and Ag-containing chemisorbent in selective acetylene hydrogenation in an ethane-ethylene mixture was studied. It was found that carbonaceous deposits are formed on dispersed silver particles of the Pd-Ag-Al2O3 catalyst and on large silver crystallites of the Ag-Al2O3 chemisorbent. The lightest rapidly burning hydrocarbons of composition CH2.5m with a low degree of aromaticity are sorbed on the silver particles or in the nearest neighborhood of them. Slowly burning hydrocarbons of composition CH1.5–1.8m with high a degree of aromaticity are fixed on the alumina support. It was suggested to replace the Pd-Ag-Al2O3 catalyst currently used at the Nizhnekamskneftekhim plant in the process of selective acetylene hydrogenation by a system with a lower content of silver or without it.
Similar content being viewed by others
References
Plate, N.A. and Slivinskii, E.V., Osnovy khimii i tekhnologii monomerov (Fundamentals of the Chemistry and Technology of Monomers), Moscow: Nauka, 2002.
Molnar, A., Sarkany, A., and Varga, M., J. Mol. Catal. A: Chem., 2001, vol. 173, p. 185.
Stacchiola, D., Calaza, F., Zheng, T., et al., J. Mol. Catal. A: Chem., 2005, vol. 228, p. 35.
Borodzinski, A., Catal. Lett., 1999, vol. 63, p. 35.
Huang, D.C., Chang, K.H., Pong, W.F., et al., Catal.: Lett., 1998, vol. 53, p. 155.
Busygin, V.M., Lamberov, A.A., Egorova, S.R., et al., Katal. Prom-sti., 2006, no. 2, p. 24.
Busygin, V.M., Lamberov, A.A., Egorova, S.R., et al., Katal. Prom-sti, 2006, no. 3, p. 34.
Bond, G.C., Appl. Catal., A: Gen., 1997, vol. 149, p. 3.
Duca, D., Barone, G., and Varga, Z., Catal. Lett., 2001, vol. 72, nos. 1–2, p. 17.
Sarkany, A., React. Kinet. Catal. Lett., 2001, vol. 74, no. 2, p. 299.
Kim, W.J., Shin, E.W., Kang, J.H., et al., Appl. Catal. A: Gen., 2003, vol. 241, p. 305.
Guisent, M. and Magnoux, P., Appl. Catal. A: Gen., 2001, vol. 212, p. 83.
Shaikhutdinov, Sh.K., Frank, M., Baumer, M., et al., J. Catal. Lett., 2002, vol. 80, nos. 3–4, p. 115.
Judari, K., Abbet, S., Worz, A.S., et al., J. Mol. Catal. A: Chem., 2003, vol. 199, p. 103.
Bartolomew, C.H., Appl. Catal., A: Gen., 2001, vol. 212, p. 17.
Sarkany, A. and Revay, Zs., Appl. Catal., A: Gen., 2003, vol. 243, p. 347.
ASTM D 3663-99 Manual Standartnyi metod opredeleniya ugleroda i sery v katalizatorakh i nositelyakh katalizatorov (ASTM D 3663-99 Manual “Standard Method of Carbon and Sulfur Determination in Catalysts and Catalyst Supports”).
Paukshtis, E.A., Infrakrasnaya spektroskopiya v geterogennom kislotno-osnovnom katalize (Infrared Spectroscopy in Heterogeneous Acid-Base Catalysis), Novosibirsk: Nauka, 1992.
Umanskii, Ya.S., Skakov, Yu.A., Ivanov, A.M., and Rastorguev, L.I., Kristallografiya, rentgenografiya i elektronnaya mikroskopiya (Crystallography, X-ray Diffraction, and Electron Microscopy), Moscow: Metallurgiya, 1982.
Kogan, S.B., Kogan, S.B., Podkletnova, N.M., et al., Zh. Prikl. Khim., 1983, vol. 56, no. 8, p. 1832.
Lamberov, A.A., Romanova, R.G., and Sitnikova, E.Yu., Katal. Prom-sti, 2003, no. 2, p. 96.
Van Krevelen, D.W. and Schuyer, J., Coal Science, Amsterdam: Elsevier. Translated under the title Nauka ob ugle, Moscow: Khimiya, 1986.
Author information
Authors and Affiliations
Additional information
Original Russian Text © S.P. Egorova, A.A. Lamberov, L.R. Galimzyanova, M.V. Nazarov, V.M. Shatilov, Kh.Kh. Gil’manov, 2009, published in Kataliz v Promyshlennosti.
Rights and permissions
About this article
Cite this article
Egorova, S.P., Lamberov, A.A., Galimzyanova, L.R. et al. Deactivation of the Pd-Ag-Al2O3 catalyst and Ag-Al2O3 chemisorbent at joint operation in the process of selective acetylene hydrogenation. Catal. Ind. 1, 102–110 (2009). https://doi.org/10.1134/S2070050409020020
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070050409020020