Abstract
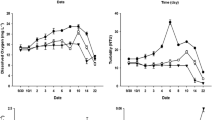
Experiments in microcosms have demonstrated that beaver vital activity products (BVAPs) promote an increase in concentrations of total nitrogen (N) and total phosphorus (P), a decrease in the N/P value in water, and an increase in the abundance and biomass of bacterioplankton. Under such conditions, the abundance and biomass of small Ceriodaphnia dubia Richard and large Daphnia (Ctenodaphnia) magna Straus, which live separately, increase. The coexistence of these cladocerans in microcosms under the BVAP influences results in a high increase in the abundance and biomass of D. magna; in similar experiments without the influence of BVAP, Ceriodaphnia dubia becomes more abundant. The results of bioassay demonstrate that the number of newborns of Ceriodaphnia dubia decreases in water where Daphnia magna is numerous owing to BVAPs. It is suggested that the vital activity products of large representatives of the genus Daphnia inhibit the fecundity of small species of Cladocera. This fact, along with the high competitiveness of large cladoceran species under conditions of a high level of nutritive base, determine the formation of zooplankton communities in beaver ponds which are characterized by a high abundance and biomass and low uniformity
Similar content being viewed by others
References
Andersen, T. and Hessen, D.O., Carbon, nitrogen, and phosphorus content of freshwater zooplankton, Limnol. Oceanogr., 1991, vol. 36, pp. 807–814.
Balushkina, E.B. and Vinberg, G.G., Dependence between weight and length of the body of planktonic animals, in Obshchie osnovy izucheniya vodnykh ekosistem (General Principles of Analysis of Aquatic Ecosystems), Leningrad: Nauka, 1979, pp. 169–172.
Bargmann, C.I., Comparative chemosensation from receptors to ecology, Nature, 2006, vol. 444, pp. 295–301.
Beklemishev, V.N., Classification of biocenological (symphysiological) links, Byull. Mosk. O-va. Ispyt. Prir., Otd. Biol., 1951, vol. 57, no. 5, pp. 3–30.
Boersma, M. and Kreutzer, C., Life at the edge: is food quality really of minor importance at low quantities? Ecology, 2002, vol. 83, no. 9, pp. 2552–2561.
Burns, C.W., Crowding-induced changes in growth, reproduction and morphology of Daphnia, Freshwater Biol., 2000, vol. 43, no. 1, pp. 19–29.
Chaichana, R., Leah, R., and Moss, B., Birds as eutrophicating agents: a nutrient budget for a small lake in a protected area, Hydrobiologia, 2010, vol. 646, pp. 111–121.
Chalova, I., Krylov, A., Shevchenko, N., and Lavrov, V., The effect concentration of beavers’ vital activity products on Cladocera fertility in laboratory experiments, 6th Int. Beaver Symp., Croatia, September 17–20, 2012, Abstracts of Papers, Zagreb: Univ. of Zagreb, 2011, p. 65.
Chemical Communication in Crustaceans, Breithaupt, T. and Thiel, M., Eds., New York: Springer-Verlag, 2010.
Chalova, I.V., Shevchenko, N.S., Tsel’movich, O.L., and Krylov, A.V., Reaction of Ceriodaphnia dubia Richard on life remains of water and near-water environmentforming species, Mezhd. shkola-konf. “Aktual’nye problemy izucheniya rakoobraznykh kontinental’nykh vod,” Borok, 5–9 noyabrya 2012 g. (Int. School-Conf. “Urgent Problems of Analysis of Crustacean in Continental Waters,” Borok, November 5–9, 2012), Kostroma: Kostromsk. Dom Pechati, 2012, pp. 309–312.
Chuikov, Yu.S., Ecological analysis of composition and structure of communities of aquatic animals. Ecological classification of invertebrates found in freshwater plankton, Ekologiya, 1981, no. 3, pp. 71–77.
Collen, P. and Gibson, R.J., The general ecology of beavers (Castor spp.), as related to their influence on stream ecosystems and riparian habitats, and subsequent effects on fish—a review, Rev. Fish Biol. Fish., 2001, vol. 10, pp. 439–461.
Ekosistema maloi reki v izmenyayushchikhsya uloviyakh sredy (Ecosystem of Small River in Changing Environmental Conditions), Moscow: KMK, 2007.
Feneva, I.Yu., Palash, A.L., and Budaev, S.V., Influence of food abundance and biotic relationships on successful introduction of large and small species of Cladocera in experiments, Zool. Zh., 2010, vol. 89, no. 4, pp. 416–423.
Gapeeva, M.V., Razgulin, S.M., and Skopintsev, B.A., Cartilage persulfate analysis of total nitrogen in natural waters, Gidrokhim. Mater., 1984, vol. 87, pp. 67–70.
Golovkin, A.N., Influence of marine colonial birds on development of phytoplankton, Okeanologiya (Moscow), 1967, vol. 4, no. 4, pp. 272–282.
Ivleva, I.V., Biologicheskie osnovy i metody massovogo kul’tivirovaniya kormovykh bespozvonochnykh (Biological Principles and Methods of Mass Cultivation of Food Invertebrates), Moscow: Nauka, 1969.
Jones, C.G., Lawton, J.H., and Shachak, M., Organisms as ecosystem engineers, Oikos, 1994, vol. 69, pp. 373–386.
Krylov, A.V., Activity of beavers as an ecological factor affecting the zooplankton of small rivers, Russ. J. Ecol., 2002, vol. 33, no. 5, pp. 349–355.
Krylov, A.V., Zooplankton ravninnykh malykh rek (Zooplankton of the Plain Small Rivers), Moscow: Nauka, 2005.
Krylov, A.V., Impact of beaver activity upon zooplankton of the small rivers in the upper Volga basin, in Restoring the European Beaver: 50 Years of Experience, Sjöberg, G. and Ball, J.P., Eds., Sofia: Pensoft, 2011, pp. 241–254.
Krylov, A.V., Chalova, I.V., and Tsel’movich, O.L., Cladocerans under conditions of small river damming by man and beavers, Russ. J. Ecol., 2007, vol. 38, no. 1, pp. 34–38.
Krylov, A.V., Kulakov, D.V., Zhalova, I.V., and Papchenkov, V.G., Zooplankton presnykh vodoemov v usloviyakh vliyaniya gidrofil’nykh ptits (Freshwater Zooplankton Affected by Hydrophilic Birds), Izhevsk: Izd. S.A. Permyakova, 2012.
Kotliar, N.B., Application of the new keystone-species concept to prairie dogs: how well does it work? Conserv. Biol., 2000, vol. 14, no. 6, pp. 1715–1721.
Lampert, W., Daphnia: Development of a Model Organism in Ecology and Evolution, Oldendorf: Int. Ecol. Inst., 2011.
Legeida, I.S., Dolinskii, V.L., and Rogozyanskaya, T.D., Influence of beaver on hydrological fauna, Gidrobiol. Zh., 1987, vol. 23, no. 6, pp. 97–98.
Legeida, I.S. and Rogozyanskaya, T.D., Zooplankton in the beaver’s habitats, Gidrobiol. Zh., 1981, vol. 17, no. 2, pp. 16–21.
Metodika opredeleniya toksichnosti vody i vodnykh vytyazhek iz pochv, osadkov stochnykh vod, otkhodov po smertnosti i izmeneniyu plodovitosti tseriodafnii (The Method for Analysis of Toxicity of Water and Water Extracts for Soils, Sediments of Waste Waters, Death Remains, and Change of Fertility of Ceriodaphnia Species), Moscow: Akvaros, 2007.
Naiman, R.J., Animal influences on ecosystem dynamics, BioScience, 1988, vol. 38, pp. 750–752.
Naiman, R.J., Melillo, J.M., and Hobbie, J.E., Ecosystem alteration of boreal forest streams by beaver (Castor canadensis), Ecology, 1986, vol. 67, no. 5, pp. 1254–1269.
Naiman, R.J., Pinay, G., Johnston, C., and Pastor, J., Beaver influence on the long-term biogeochemical characteristics of boreal forest drainage networks, Ecology, 1994, vol. 74, no. 4, pp. 905–921.
Novikov, M.A. and Kharlamova, M.N., Transbiotic factors in aquatic environment: a review, Zh. Obshch. Biol., 2000, vol. 61, no. 1, pp. 22–46.
Pain, R.T., A note on trophic complexity and community stability, Am. Nat., 1969, vol. 103, pp. 91–93.
PND F 14.1:2.106-97. Metodika vypolneniya iznerenii massovoi kontsentratsii fosfora obshchego v probakh prirodnykh i ochishchennykh stochnykh vod fotometricheskim metodom posle okisleniya persul’fatom (PND F 14.1:2.106-97: Measurement of Mass Concentration of Total Phosphorous in Natural and Purified Waste Water Probes by Photometry after Oxidation Using Persulphate), Moscow: Akvaros, 2004.
Porter, K.G. and Feig, Y.S., The use of DAPI for identifying and counting aquatic microflora, Limnol. Oceanogr., 1980, vol. 25, no. 5, pp. 943–948.
Power, M.E., Tilman, D., Estes, J.A., Menge, B.A., Bond, W.J., Scott Mills, L., Dayly, G., Castilla J.C., Lubcenko, J., and Paine, R., Challenges in quest for keystones, BioScience, 1996, vol. 45, no. 8, pp. 609–620.
Restoring the European Beaver: 50 Years of Experience, Sjöberg, G. and Ball, J.P., Eds., Sofia: Pensoft, 2011.
Roman, J. and McCarthy, J.J., The whale pump: marine mammals enhance primary productivity in a coastal basin, PLoS One, 2010, vol. 5, no. 10, p. e13255. doi 10.1371/journal.pone.0013255
Romanovsky, Yu.E. and Feniova, I.Yu., Competition among Cladocera: effect of different levels of food supply, Oikos, 1985, vol. 44, pp. 243–252.
Rosell, F., Borzér, O., Collen, P., and Parker, H., Ecological impact of beavers Castor fiber and Castor canadensis and their ability to modify ecosystems, Mamm. Rev., 2005, vol. 35, no. 3, 4, pp. 248–276.
Tolomeev, A.P., A concept “ecological stoichiometry” in aquatic ecosystems: literature review, Sib. Ekol. Zh., 2006, no. 1, pp. 13–19.
Townsend, C.R., The patch dynamics concept of stream community ecology, J. North Am. Benthol. Soc., 1989, vol. 8, pp. 36–50.
Urabe, J., Clasen, J., and Sterner, R.W, Phosphorus limitation of Daphnia growth: is it real, Limnol. Oceanogr., 1997, vol. 42, no. 6, pp. 1436–1443.
Zadereev, E.S., Chemical interactions among planktonic crustaceans, Zh. Obshch. Biol., 2002, no. 2, pp. 149–157.
Zavyalov, N.A., Krylov, A.V., Bobrov, A.A., Ivanov, V.K., and Dgebuadze, Yu.Yu., Vliyanie rechnogo bobra na ekositemy malykh rek (Influence of River Beaver on Small River Ecosystems), Moscow: Nauka, 2005.
Zherikhin, V.V., Evolutionary biocenology: a problem of model choice, in Ekosistemnye perestriki i evolyutsiya biosfery (Ecosystem Transformations and Evolution of Biosphere), Moscow: Nedra, 1994, pp. 13–20.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.V. Krylov, I.V. Chalova, N.S. Lapeeva, O.L. Tselmovich, A.V. Romanenko, V.L. Lavrov, 2016, published in Sibirskii Ekologicheskii Zhurnal, 2016, No. 4, pp. 600–610.
Rights and permissions
About this article
Cite this article
Krylov, A.V., Chalova, I.V., Lapeeva, N.S. et al. Experimental studies of the effect of beaver (Castor fiber L.) vital activity products on the formation of zooplankton structure (by the example of growth of two cladoceran species of different sizes). Contemp. Probl. Ecol. 9, 494–502 (2016). https://doi.org/10.1134/S1995425516040089
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995425516040089