Abstract
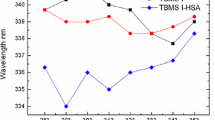
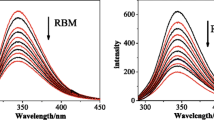
The aim of this present work is to investigate the interaction between doxorubicin and bovine serum albumin (BSA) in simulated physiological conditions by spectroscopic methods to reveal potential toxic effects of the drug. The results reflected that doxorubicin made the fluorescence quenching of BSA through a static quenching procedure. The binding constants at 293, 298, and 303 K were obtained as 2.53 × 105, 8.13 × 104, and 3.59 × 104 M–1, respectively. There may be one binding site of doxorubicin on BSA. The thermodynamic parameters indicated that the interaction between doxorubicin and BSA was driven mainly by hydrogen bonding and electrostatic forces. Synchronous fluorescence spectra and circular dichroism (CD) results showed doxorubicin binding slightly changed the conformation of BSA with secondary structural content changes. Förster resonance energy transfer (FRET) study revealed high possibility of energy transfer with doxorubicin-Trp-212 distance of 3.48 nm. The results of the present study may provide valuable information for studying the distribution, toxicological and pharmacological mechanisms of doxorubicin in vivo.
Similar content being viewed by others
References
D. Charbonneau, M. Beauregard, and H. A. Tajmir-Riahi, J. Phys. Chem. B 113, 1777 (2009).
D. M. Charbonneau and H. A. TajmirRiahi, J. Phys. Chem. B 114, 1148 (2010).
X. X. Cheng, Y. Lui, B. Zhou, X. H. Xiao, and Y. Liu, Spectrochim. Acta A: Mol. Biomol. Spectrosc. 72, 922 (2009).
X. C. Zhao and R. T. Liu, Environ. Int. 40, 244 (2012).
R. Beauchemin, C. N. N’SoukpoeKossi, T. J. Thomas, T. Thomas, R. Carpentier, et al., Biomacromolecules 8, 3177 (2007).
Z. Cheng, J. Pharmaceut. Biomed. Anal. 66, 240 (2012).
C. Tan, H. Tasaka, K. P. Yu, M. L. Murphy, and D. A. Karnofsky, Cancer 20, 333 (1967).
A. Dimarco, M. Gaetani, and B. Scarpina, Cancer Chemother. Rep. Pt. 1 53, 33 (1969).
A. Varlan, S. Ionescu, and M. Hillebrand, Lumines-cence 26, 710 (2011).
X. Zhao, F. Sheng, J. Zheng, and R. Liu, J. Agricult. Food Chem. 59, 7902 (2011).
A. B. Khan, J. M. Khan, M. S. Ali, R. H. Khan, and K.U. Din, Colloids Surf., B 87, 447 (2011).
F. Xu, L. Zhang, L. He, W. Gu, F. Fang, et al., Acta Chim. Sin. 69, 2228 (2011).
A. Bolli, M. Marino, G. Rimbach, G. Fanali, M. Fasano, et al., Biochem. Biophys. Res. Commun. 398, 444 (2010).
B. H. M. Hussein, J. Luminesc. 131, 900 (2011).
J. S. Mandeville and H. A. TajmirRiahi, Biomacro-molecules 11, 465 (2010).
D. W. Lu, X. C. Zhao, Y. C. Zhao, B. C. Zhang, B. Zhang, et al., Food Chem. Toxicol. 49, 3158 (2011).
H. Bian, M. Li, Q. Yu, Z. Chen, J. Tian, et al., Int. J. Biol. Macromol. 39, 291 (2006).
Y. J. Hu, Y. Liu, and X. H. Xiao, Biomacromolecules 10, 517 (2009).
X. Zhao, R. Liu, Y. Teng, and X. Liu, Sci. Total Envi-ron. 409, 892 (2011).
C. N. Yan, H. X. Zhang, P. Mei, and Y. Liu, Chin. J. Chem. 23, 1151 (2005).
R. Subramanyam, A. Gollapudi, P. Bonigala, M. Chin-naboina, and D. G. Amooru, J. Photochem. Photo-biol., B 94, 8 (2009).
J. N. Tian, J. Q. Liu, W. Y. He, Z. D. Hu, X. J. Yao, et al., Biomacromolecules 5, 1956 (2004).
J. N. Tian, J. Q. Liu, Z. Hu, and X. G. Chen, Bioorg. Med. Chem. 13, 4124 (2005).
S. M. T. Shaikh, J. Seetharamappa, P. B. Kandagal, D. H. Manjunatha, and S. Ashoka, Dyes Pigments 74, 665 (2007).
X. C. Zhao, R. T. Liu, Z. X. Chi, Y. Teng, and P. F. Qin, J. Phys. Chem. B 114, 5625 (2010).
N. Abdollahpour, A. Asoodeh, M. R. Saberi, and J. Chamani, J. Luminesc. 131, 1885 (2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Xi, A., Xu, Z., Liu, F. et al. Toxic effects of anticancer drug doxorubicin to bovine serum albumin evaluated by spectroscopic methods. Russ. J. Phys. Chem. B 9, 946–951 (2015). https://doi.org/10.1134/S1990793115060238
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793115060238