Abstract
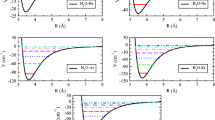
The results of an investigation of the influence of isobaric heating on distribution of hydrogen bonds in the H2O-D2O system on the basis of comparative complex analysis of the v(OD) and 2v 3(H2O) spectral bands are presented. In the case of analysis of the band assigned to the 2v 3(H2O) overtone, the possibilities of obtaining more detailed information on the influence of external factors on the formation of local water structure widen significantly. In particular, there are noticeable differences in the quality of the obtainable data for these bands, which are especially manifested in their low-frequency regions, which are determined by the presence of strong hydrogen bonds characteristic of H-bonded high order water n-mers.
Similar content being viewed by others
References
Y. E. Gorbaty and G. V. Bondarenko, Appl. Spectrosc. 53, 908, (1999).
Yu. E. Gorbaty, Sverkhkrit. Fluidy Teor. Prakt. 2 (1), 40 (2007).
R. D. Oparin, in Problems of Solution Chemistry. Teoretical and Experimental Methods of Solution Chemistry (Prospekt, Moscow, 2011), p. 255 [in Russian].
R. Oparin, T. Tassaing, Y. Danten, and M. Besnard, J. Chem. Phys. 123, 224501 (2005).
R. Oparin, T. Tassaing, Y. Danten, and M. Besnard, J. Chem. Phys. 120, 10691 (2004).
R. D. Oparin and M. V. Fedotova, Russ. J. Gen. Chem. 77, 17 (2007).
R. D. Oparin and M. V. Fedotova, Russ. J. Gen. Chem. 77, 1686 (2007).
G. Herzberg, in Molecular Spectra and Molecular Structure: Infrared and Raman Spectra of Polyatomic Molecules (Van Nostrand, Princeton, 1956), Vol. 2, p. 273.
M. Buback, Zeitschr. Naturforsch. A 39, 399 (1984).
M. Buback, J. Schweer, and H. Tups, Zeitschr. Naturforsch. A 41, 505 (1986).
M. Buback, J. Schweer, and H. Tups, Zeitschr. Naturforsch. A 41, 512 (1986).
H. G. Kjaergaard, H. Wei, S. Lefebvre, T. Carrington, O. S. Mortensen, M. L. Sage, and B. R. Henry, J. Chem. Phys. 100, 6228 (1994).
R. D. Oparin, Sverkhkrit. Fluidy Teor. Prakt. 7 (2), 38 (2012).
A. H. Narten, M. D. Danford, and H. A. Levy, ORNL-3997 (1966), pp. 1–67.
Y. E. Gorbaty and Y. N. Demianets, Chem. Phys. Lett. 100, 450 (1983).
Z. S. Klemenkova, T. A. Novskova, and A. K. Lyashchenko, Russ. J. Phys. Chem. A 82, 571 (2008).
E. Stanley, J. Phys. A: Math. Gen. 12 (12), L329 (1979).
E. Stanley and J. J. Teixeira, Chem. Phys. 73, 3404 (1980).
E. Stanley, J. J. Teixeira, A. Geiger, and R. L. Blumberg, Physica A 106, 981 (1981).
G. V. Bondarenko and Yu. E. Gorbaty, Dokl. Akad. Nauk SSSR 210 (1), 132 (1973).
P. R. Griffiths, in Laboratory Methods in Vibrational Spectroscopy (Wiley, Chichester, England, 1987), p. 121.
D. A. Sirotkin, Candidate's Dissertation in Chemistry (Moscow, 2004).
M. Iwamoto, J. Uozumi, and K. Nishinari, in Proceedings of the International NIR/NIT Conference (Akademiai Kiado, Budapest, Hungary, 1986), p. 3.
H. Maeda, Y. Ozaki, M. Tanaka, N. Hayashi, and T. Kojima, J. Near Infrared Spectrosc. 3, 191 (1995).
V. H. Segtnan, S. Sasik, T. Isaksson, and Y. Ozaki, Anal. Chem. 73, 3153 (2001).
I. Noda, Appl. Spectrosc. 44, 550 (1990).
I. Noda, Appl. Spectrosc. 47, 1329 (1993).
I. Noda, A. E. Dowrey, C. Marcott, G. M. Story, and Y. Ozaki, Appl. Spectrosc. 54 (7), 236A (2000).
M. E. Wall, A. Rechtsteiner, and L. M. Rocha, in A Practical Approach to Microarray Data Analysis (Kluwer, Norwell, MA, 2003), p. 91.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oparin, R.D., Dyshin, A.A. & Kiselev, M.G. Comparative analysis of the v(OD) and 2v 3(H2O) spectral bands of H2O-D2O system under isobaric heating conditions. Russ. J. Phys. Chem. B 7, 863–879 (2013). https://doi.org/10.1134/S1990793113070129
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793113070129