Abstract
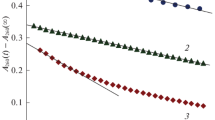
Ozone decomposition in pure water, H2O2 aqueous solution, and water with ethanol admixture is investigated. It is found that, in the presence of H2O2, ozone decomposes significantly faster than in pure water owing to its interaction with H2O2. The effective rate constant (k = (0.023 ± 0.002) l/(mol s)) is determined, which is much higher than the overall rate constant of ozone decomposition in water, but much lower than the specific rate of ozone decomposition in water in the presence of common organic admixtures (fulvic acids, ethanol). It is concluded that at the combined use of O3 and H2O2 for the decomposition of organic admixtures in water, the contribution of their interaction with each other is little.
Similar content being viewed by others
References
Yu. I. Skurlatov, Usp. Khim. 60(3), 575 (1991).
Ozone and Other Ecologically Pure Oxidants. Science and Engineering. Proceedings of the 30th All-Russia Seminar, Moscow (MAKS Press, Moscow, 2008).
V. A. Petrenev, Tsellyul. Bumaga Karton, Nos. 1–2, 14 (1996).
T. R. Card and R. L. Purdom, in Ozone World Congr., Ozone in Wastewater Treatment (New York, 1989), vol. 2, p. 281.
G. Reynolds, N. Graham, A. Perry, and R. G. Rice, Ozone Sci. Eng. 11, 339 (1989).
W. H. Glaze and J.-W. Kang, Ind. Eng. Chem. Res. 28, 1573 (1989).
S. D. Razumovskii, Russ. J. Phys. Chem. A 70, 1237 (1996).
Ozone and Methods of Efferent Therapy in Medicine, Proceedings, VINITI, News of Science and Engineering, Ser. Medicine (Moscow, 2000) [in Russian].
T. G. Shcherbatyuk, Candidate’s Dissertation in Medical Science (Nizhni Novgorod Medical Academy, Nizhni Novgorod, 1997).
S. D. Razumovskii, M. L. Konstantinova, and V. Ya. Zaitsev, Biomed. Khim. 56, 380 (2010).
S. D. Razumovskii, M. L. Konstantinova, T. V. Grinevich, and G. V. Korovina, Kinet. Catal. 51, 492 (2010).
B. G. Ershov and P. A. Morozov, Russ. J. Phys. Chem. A 83, 1295 (2009).
U. von Gutten, Water Res. 37, 1443 (2003).
K. Sehested, H. Gorfitzen, J. Holcman, H. Fischer, and E. J. Hart, Environ. Sci. Res. 25, 1589 (1991).
M. L. Konstaninova, S. D. Razumovskii, and G. E. Zaikov, Izv. Akad. Nauk, Ser. Khim., No. 6, 1443 (1992).
S. D. Razumovskii and G. E. Zaikov, Ozone and its Reactions with Organic Compounds (Nauka, Moscow, 1974), p. 214 [in Russian].
S. D. Razumovskii, M. L. Konstantinova, and V. V. Podmaster’ev, Izv. Akad. Nauk, Ser. Khim., No. 1, 60 (1996).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © S.D. Razumovskii, T.V. Grinevich, G.V. Korovina, 2011, published in Khimicheskaya Fizika, 2011, Vol. 30, No. 10, pp. 54–56.
Rights and permissions
About this article
Cite this article
Razumovskii, S.D., Grinevich, T.V. & Korovina, G.V. Reactivity of hydrogen peroxide with respect to ozone. Russ. J. Phys. Chem. B 5, 797–799 (2011). https://doi.org/10.1134/S1990793111090211
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793111090211