Abstract
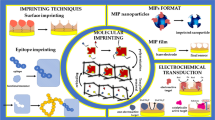
The review summarizes current knowledge on the main approaches used for creation of high affinity polymer analogs of antibodies (known as molecularly imprinted polymers, MIP) applicable for electroanalysis of functionally important proteins such as myoglobin, troponin T, albumin, ferritin, lysozyme, calmodulin. The main types of monomers for MIP preparation as well as methods convenient for analysis of MIP/protein interactions, such as surface plasmon resonance (SPR), nanogravimetry with the use of a quartz crystal resonator (QCM), spectral and electrochemical methods have been considered. Special attention is paid to experimental data on electrochemical registration of myoglobin by means of o-phenylenediaminebased MIP electrodes. It was shown that the imprinting factor calculated as a ratio of the myoglobin signal obtained after myoglobin insertion in MIP to the myoglobin signal obtained after myoglobin insertion in the polymer lacking the molecular template (NIP) is 2–4.
Similar content being viewed by others
Abbreviations
- CV:
-
cyclic voltammetry
- DPV:
-
differential pulse voltammetry
- EIS:
-
electrochemical impedance spectroscopy
- QCM:
-
quartz crystal microbalance
- SPR:
-
surface plasmon resonance
- SWV:
-
square wave voltammetry
References
Archakov, A., Ivanov, Yu., Lisitsa, A., and Zgoda, V., Proteomics, 2009, vol. 9, pp. 1326–1343.
Deev, S.M. and Lebedenko, E.N., Acta Naturae, 2009, vol. 1, pp. 32–50.
Barié, N. and Rapp, M., Biosens. Bioelectron., 2001, vol. 16, pp. 979–987.
Petrovskaya, L.E., Shingarova, D.A., Dolgikh, M.P., and Kirpichnikov, M.P., Bioorgan. Khim., 2011, vol. 37, pp. 581–591.
Radom, F., Jurek, P.M., Mazurek, M.P., Otlewski, J., and Jeleń, F., Biotechnol. Advances, 2013, vol. 31, pp. 1260–1274.
Radko, S., Rakhmetova, S., Bodoev, N., and Archakov, A., Biochemistry (Moscow) Supplement Series B: Biomedical Chemistry, 2007, vol. 1, pp. 198–209.
Spiridonova, V.A., Biomed. Khim., 2010, vol. 56, pp. 639–656.
Shcherbinin, D.S. and Veselovsky, A.V., Biofizika, 2013, vol. 58, pp. 415–424.
Gendrikson, O.D., Zherdev, A.V., and Dzantiev, B.B., Usp. Biol. Khim., 2006, vol. 46, pp. 149–192.
Li, S., Cao, S., Whicombe, M., and Piletsky, S., Progress in Polymer Science, 2014, vol. 39, pp. 145–163.
Polyakov, M.V., Zhur. Fiz. Khim., 1931, vol. 2, pp. 799–805.
Polyakov, M.V., Kuleshina, L., and Neimark, I., Zhur. Fiz. Khim., 1937, vol. 10, pp. 100–112.
Dickey, F.H., Proc. Natl. Acad. Sci. USA, 1949, vol. 35, pp. 227–229.
Pauling, L., J. Am. Chem. Soc., 1940, vol. 62, pp. 2643–2657.
Wulff, G. and Sarhan, A., Angew. Chem. Int. Ed., 1972, vol. 11, pp. 341–348.
Yaqub, S., Latif, U., and Dickert, F.L., Sensors and Actuators B, 2011, vol. 160, pp. 227–233.
Verheyen, E., Schillemans, J.P., van Wijk, M., Demeniex, M.A., Hennink, W.E., and van Nostrum, C.F., Biomaterials, 2011, vol. 32, pp. 3008–3020.
Lieberzeit, P.A., Glanznig, G., Jenik, M., Gazda-Miarecka, S., Dickert, F.L., and Leidl, A. Sensors, 2005, vol. 5, pp. 509–518.
Kima, J.-M., Leea, U.-H., Changa, S.-M., and Park, J.Y., Sensors and Actuators B, 2014, vol. 200, pp. 25–30.
Liu, R., Sha, M., Jiang, S., Luo, J., and Liu, X., Talanta, 2014, vol. 120, pp. 76–83.
Yola, M.L., Uzun, L., Özaltιn, N., and Denizli, A., Talanta, 2014, vol. 120, pp. 318–324.
Cai, D., Ren, L., Zhao, H., Xu, C., Zhang, L., Yu, Y., Wang, H., Lan, Y., Roberts, M.F., Chuang, J.H., Naughton, M.J., Ren, Z., and Chiles, T.C., Nat. Nanotechnol., 2010, vol. 5, pp. 597–601.
Lv, Y., Biotechnol. Advances, 2013, vol. 31, pp. 1172–1186.
Moreira, F.T., Dutra, R.A., Noronha, J.P., and Sales, M.G., Biosens. Bioelectron., 2011, vol. 26, pp. 4760–4766.
Li, W.K. and Li, S.J., Adv. Polym. Sci., 2007, vol. 206, pp. 191–210.
Kim, J.-M., Lee, U.-H., Chang, S.-M., and Park, J.Y., Sensors and Actuators B, 2014, vol. 200, pp. 25–30.
Malitesta, C., Mazzotta, E., Picca, R.A., Poma, A., Chianella, I., and Piletsky, S.A., Anal. Bioanal. Chem., 2012, vol. 402, pp. 1827–1846.
Zhang, X., Peng, Y., Bai, J., Ning, B., Sun, S., Hong, X., Liu, Y., Liu, Y., and Gaom Z., Sensors and Actuators B, 2014, vol. 200, pp. 69–75.
Tuyen, D., Quan, D., Binh, N., Nguyen, V., Lam, T., Huyen, L., Nguen, L., Viet, P., Loc, N., and Huy, T., J. Mol. Liquids, 2014, vol. 198, pp. 307–312.
Luo, J., Jiang, S., and Liu, X., Sensors and Actuators B, 2014, vol. 203, pp. 782–789.
Choong, C.L., Bendall, J.S., and Milne, W.I., Biosens. Bioelectron., 2009, vol. 25, pp. 652–656.
Liu, Y. and Kumar, S., ACS Appl. Mater. Interfaces, 2014, vol. 6, pp. 6069–6087.
Matsunaga, T., Hishiya, T., and Takeuchi, T., Anal. Chim. Acta, 2007, vol. 591, pp. 63–67.
Kimhi, O. and Bianco-Peled, H., Langmuir, 2007, vol. 23, pp. 6329–6335.
Hawkins, D.M., Stevenson, D., and Reddy, S.M., Anal. Chim. Acta, 2005, vol. 542, pp. 61–65.
Wang, Y. and Wei, T.-X., Chem. Lett., 2013, vol. 24, pp. 813–816.
Qureshi, A., Gurbuz, Y., and Niazi, J.H., Sensors and Actuators B-Chemical, 2012, vol. 171, pp. 62–76.
Mohammed, M. and Desmulliez, M., Lab on a Chip, 2011, vol. 11, pp. 569–595.
McDonnell, B., Hearty, S., Leonard, P., and O’Kennedy, R., Clin. Biochem., 2009, vol. 42, pp. 549–561.
Karimian, N., Vagin, M., Hossein, M., Zavar, A., Chamsaz, M., Turner, A., and Tiwari, A., Biosens. Bioelectron., 2013, vol. 50, pp. 492–498.
Karimian, N., Turner, A., and Tiwari, A., Biosens. Bioelectron., 2014, vol. 59, pp. 160–165.
Moreira, F., Sharma, S., Dutra, R., Noronha, J., Cass, A., and Sales, M., Biosens. Bioelectron., 2013, vol. 45, pp. 237–244.
Moreira, F., Sharma, S., Dutra, R., Noronha, J., Cass, A., and Sales, M., Sensors and Actuators B, 2014, vol. 196, pp. 123–132.
Bueno, L., El-Sharif, H.F., Salles, M.O., Boehm, R.D., Narayan, R.J., Paixão, T.R.L.C., and Reddy, S.M., Sensors and Actuators B, 2014, vol. 204, pp. 88–95.
Shumyantseva, V.V., Bulko, T.V., Suprun, E.V., Kuzikov, A.V., Agafonova, L.E. and Archakov, A.I., Biomed. Khim., 2015, vol. 61, pp. 185–204.
Sharma, P., Iskierko, Z., Pietrzyk-Le, A., D’Souza, F., and Kutner, W., Electrochemistry Communications, 2015, vol. 50, pp. 81–87.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.V. Shumyantseva, T.V. Bulko, I.H. Baychorov, A.I. Archakov, 2016, published in Biomeditsinskaya Khimiya.
Rights and permissions
About this article
Cite this article
Shumyantseva, V.V., Bulko, T.V., Baychorov, I.H. et al. Molecularly imprinted polymers (MIP) in electroanalysis of proteins. Biochem. Moscow Suppl. Ser. B 10, 145–151 (2016). https://doi.org/10.1134/S1990750816020104
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990750816020104