Abstract
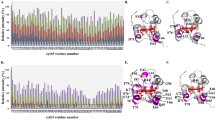
The interaction sites for protein partners, cytochrome P450 2B4 (d-2B4) and NADPH: cytochrome P450 reductase (d-Fp), have been identified. These proteins form complexes during their functioning. Nonspecific covalent cross-linking of the d-2B4 complexes with d-Fp in the Emulgen 913 monomerized system was achieved by 4,4′- dithiobis-phenyl azide. Covalently cross-linked peptides of this complex were identified by ESI-MS/MS. Several binding sites have been identified for these proteins. Based on these sites a model for intermolecular interaction between these proteins has been proposed. This model includes 5 contact sites on d-2B4 for d-Fp (stabilized by the cross-linker); these include the following pairs of corresponding peptides of d-2B4 and d-Fp: 1) d-2B4324–336 and d-Fp570–585; 2) d-2B4423–433 and d-Fp102–109; 3) d-2B4327–336 and d Fp452–464; 4) d-2B4192–197 and d-Fp456–464; 5) d-2B4134–139 and d-Fp406–425. In the two last cases d-Fp peptides are located in the interdomain cleft and stabilize the protein-protein complex via the cross-linker and so the d 2B4/d-Fp complex formation by these sites may involve amino acid residues of the peptides d-Fp456–464 and d-Fp406–425, which surround the interdomain cleft.
Similar content being viewed by others
Abbreviations
- d-2B4:
-
cytochrome P450 2B4
- d-b5:
-
cytochrome b5
- d-Fp:
-
NADPH cytochrome P450 reductase
- DTBPA:
-
4,4′-dithiobis-phenyl azide
- KP/E:
-
potassium phosphate buffer containing 0.25 g/l Emulgen 913
References
Porter, T.D. and Coon, M.J., J. Biol. Chem., 1991, vol. 266, pp. 13469–13472.
Gotoh, O., J. Biol. Chem., 1992, vol. 267, pp. 83–90.
Guengerich, F.P., in Cytochrome P450. Enzyme Systems that Metabolise Drugs and Other Xenobiotics, Ioannides, C. Ed., John Wiley Sons Ltd., 2001.
Archakov, A.I. and Bachmanova, G.P., Cytochrome P450 and Active Oxygen, N.Y.: Taylor and Francis, 1990.
Imai, Y., J. Biochem., 1981, vol. 89, pp. 351–362.
Mayuzumi, H., Shimizu, T., Sambongi, C., Hiroya, K., and Hatano, M., Arch. Biochem. Biophys., 1994, vol. 310, pp. 367–372.
Gruenke, L.D., Konopka, K., Cadieu, M., and Waskell, L., J. Biol. Chem., 1995, vol. 270, pp. 24707–24718.
Vergeres, G. and Waskell, L., Biochimie (Paris), 1995, vol. 77, pp. 604–620.
Wang, M., Roberts, D.L., Paschke, R., Shea, T.M., Masters, B.S.S., and Kim, J.-J.P., Proc. Natl. Acad. Sci. USA., 1997, vol. 94, pp. 8411–8416.
Dean, W.L. and Gray, R.D., J. Biol. Chem., 1982, vol. 257, pp. 14679–14685.
Wagner, S.L., Dean, W.L., and Gray, R.D., J. Biol. Chem., 1984, vol. 259, pp. 2390–2395.
Lu, A.Y., Levin, W., and Kuntzman, R., Biochem. Biophys. Res. Commun., 1974, vol. 60, pp. 266–272.
Kanaeva, I.P., Dedinskii, I.R., Skotselyas, E.D., Krainev, A.G., Guleva, I.V., Sevryukova, I.F., Koen, Y.M., Kuznetsova, G.P., Bachmanova, G.I., and Archakov, A.I., Arch. Biochem. Biophys., 1992, vol. 298, pp. 395–402.
Kanaeva, I.P., Nikityuk, O.V., Davydov, D.R., Dedinskii, I.R., Koen, Y.M., Kuznetsova, G.P., Scotselyas, E.D., Bachmanova, G.I., and Archakov, A.I., Arch. Biochem. Biophys., 1992, vol. 298, pp. 403–412.
Chang, Y.-T., Stiffelman, O.B., Vakser, I.A., Loew, G.H., Bridges, A., and Waskell, L., Protein Engineering, 1997, vol. 10, pp. 119–129.
Scott, E.E., He, Y.A., Wester, M.R., White, M.A., Chin, C.C., Halpert, J.R., Johnson, E.F., and Stout, C.D., PNAS, 2003, vol. 100, pp. 13196–13201.
Shen, A.L. and Kasper, C.B., J. Biol. Chem., 1995, vol. 270, pp. 27475–27480.
Lehnerer, M., Schulze, J., Achterhold, K., Lewis, D.F., and Hlavica, P., J. Biochem. (Tokyo), 2000, vol. 127, pp. 163–169.
Bridges, A., Gruenke, L., Chang, Y.-T., Vakser, I.A., Loew, G., and Waskell, L., J. Biol. Chem., 1998, vol. 273, pp. 17036–17049.
Tamburini, P.P. and Schenkman, J.B., Mol. Pharmacol., 1986, vol. 30, pp. 178–185.
Voznesensky, A.I. and Schenkman, J.B., J. Biol. Chem., 1992, vol. 267, pp. 14669–14676.
Ivanov, Yu.D., Kanaeva, I.P., Kuznetsov, V.Yu., Lehnerer, M., Schulze, J., Hlavica, P., and Archakov, A.I., Arch. Biochem. Biophys., 1999, vol. 362, pp. 87–93.
Ivanov, Yu.D., Kanaeva, I.P., Gnedenko, O.V., Pozdnev, V.F., Shumyantseva, V.V., Samenkova, N.F., Kuznetsova, G.P., Tereza, A.M., Schmid, R.D., and Archakov, A.I., J. Mol. Recognit., 2001, vol. 14, pp. 185–196.
Muller, E.-C., Lapko, A., Otto, A., Muller, J.J., Ruckpaul, K., and Heinemann, U., Eur. J. Biochem., 2001, vol. 268, pp. 1837–1843.
Gao, Q., Doneanu, C.E., Shaffer, S.A., Adman, E.T., Goodlett, D.R., and Nelson, S.D., J. Biol. Chem., 2006, vol. 281, pp. 20404–20417.
Mikkelsen, R.B. and Wallach, D.F., J. Biol. Chem., 1976, vol. 251, pp. 7413–7416.
Dentler, W.L., Pratt, M.M., and Stephens, R.E., J. Cell. Biol., 1980, vol. 84, pp. 381–403.
Zakowski, J.J. and Wagner, R.R., J. Virol., 1980, vol. 36, pp. 93–102.
Meisenheimer, K.M. and Koch, T.H., Critical Reviews in Biochem. and Mol. Biol., 1997, vol. 32(2), pp. 101–140.
Karuzina, I.I., Zgoda, V.G., Kuznetsova, G.P., Samenkova, N.F., and Archakov, A.I., Free Radical Biology and Medicine, 1999, vol. 26, pp. 620–632.
Kanaeva, I.P., Skotselyas, E.D., Kuznetsova, G.P., Antonova, G.N., and Bachmanova, G.I., Biokhimiya, 1985, vol. 50, pp. 1382–1388.
Spatz, L. and Strittmatter, P., Proc. Natl. Acad. Sci. USA, 1971, vol. 68, pp. 1042–1046.
Laemmli, U.K., Nature, 1970, vol. 227, pp. 680–685.
Shevchenko, A., Keller, P., Scheiffele, P., Mann, M., and Simons, K., Electrophoresis, 1997, vol. 18, pp. 2591–2600.
Kuznetsov, V.Yu., Ivanov, Yu.D., and Archakov, A.I., Proteomics, 2004, vol. 4, pp. 2390–2396
Ivanov, Yu.D., Ivanov, A.V., Petushkova, N.A., Gara, O.G., Kuznetsov, V.Yu., Podoplelov, A.V., and Archakov, A.I., Biomed. Khim. 2008, vol. 54, pp. 435–444.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.V. Ivanov, A.T. Kopylov, V.G. Zgoda, I.Yu. Toropygin, E.V. Khryapova, Yu.D. Ivanov, 2009, published in Biomeditsinskaya Khimiya.
Rights and permissions
About this article
Cite this article
Ivanov, A.V., Kopylov, A.T., Zgoda, V.G. et al. Mass spectrometry identification of cytochrome P450 2B4 interaction sites for NADPH: Cytochrome P450 reductase. Biochem. Moscow Suppl. Ser. B 3, 361–371 (2009). https://doi.org/10.1134/S1990750809040052
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990750809040052