Abstract
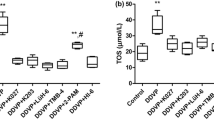
Isoform H of acetylcholinesterase (AChE) is located on the outer surface of erythrocytes; its inhibition with organophosphorus compounds (OPC) may impair structural and functional properties of erythrocytes. The aim of this study was to elucidate the effects of paraoxon (POX) on the osmotic resistance, the level of reactive oxygen species (ROS), intracellular esterase activity, and externalization of phosphatidylserine in rat erythrocytes under oxidative stress induced by tert-butyl hydroperoxide (tBH). It has been found that POX did not affect the level of ROS and the activity of intracellular esterases; however, under conditions of induced oxidative stress, it potentiated the effects associated with impairment of the erythrocyte deformation characteristics and provoked cell death associated with externalization of phosphatidylserine. The effects of POX were not caused by the solvent DMSO and were observed only in the calcium containing medium. The results obtained with the in vitro model on the combined effects of the primary specific and secondary nonspecific factors on erythrocytes may be of help in the development or improvement of combined therapeutics for acute poisonings and the prevention of their consequences.




Similar content being viewed by others
REFERENCES
Daniels G. 2007. Functions of red cell surface proteins. Vox Sang. 93 (4), 331–340.
Officioso A., Manna C., Alzoubi K., Lang F. 2016. Bromfenvinphos induced suicidal death of human erythrocytes. Pestic Biochem. Physiol. 126, 58–63.
Szatkowska B., Bukowska B., Huras B. 2011. The effect of bromfenvinphos and its impurities on human erythrocyte. Food Chem. Toxicol. 49 (2), 502–507.
Sosnowska B., Huras B., Bukowska B. 2015. Oxidative stress in human erythrocytes treated with bromfenvinphos and its impurities. Pestic Biochem. Physiol. 118, 43–49.
Santos N.C., Figueira-Coelho J., Saldanha C., Martins-Silva J. 2002. Biochemical, biophysical and haemorheological effects of dimethylsulphoxide on human erythrocyte calcium loading. Cell Calcium. 31 (4), 183–188.
Goncharov N.V., Prokofieva D.S., Voitenko N.G., Babakov V.N., Glashkina L.M. 2010. Molecular mechanisms of cholinergic regulation and dysregulation. Toksikol. vestnik (Rus.). 2, 5–10.
Nadeev, A.D., Zinchenko V.P., Avdonin, P.V., Goncharov N.V. 2014. Toxic and signaling effects of reactive oxygen species. Toksikologicheskiy vestnik (Rus.). 2, 22–27.
Mandal D., Moitra P.K., Saha S., Basu J. 2002. Caspase 3 regulates phosphatidylserine externalization and phagocytosis of oxidatively stressed erythrocytes. FEBS Lett. 513 (2–3), 184–188.
Iuchi Y. 2012. Anemia caused by oxidative stress, anemia. Dr. Donald Silverberg (Ed.), InTech, Available from: http://www.intechopen.com/books/anemia/anemia-caused-byoxidative-stress.
Mindukshev I.V., Roukoyatkina N.I., Dobrilko I.A., Skwirczynskaya E.A., Nikitina E.R., Krivoshlyk V.V., Gambaryan S.P., Krivchenko A.I. 2013. Features of apoptosis of nuclear-free cells: Platelets and erythrocytes. Ross. Fisiol. zhurnal (Rus.). 99 (1), 92–110.
Walski T., Chludzińska L., Komorowska M., Witkiewicz W. 2014. Individual osmotic fragility distribution: A new parameter for determination of the osmotic properties of human red blood cells. Biomed. Res. Int. doi https://doi.org/10.1155/2014/162102
Benesch R.E., Benesch R., Yung S. 1973. Equations for the spectrophotometric analysis of hemoglobin mixtures. Anal. Biochem. 55, 245–248.
Mindukshev I.V., Krivoshlyk V.V., Dobrilko I.A., Goncharov N.V. Vivulenets E.V., Kuznetsov S.V., Krivchenko A.I. 2010. Violation of deformation and transport characteristics of erythrocytes in the development of apoptosis. Biol. membrany (Rus.). 27 (1), 28–38.
Mindukshev I.V., Senchenkova E.Y., Goncharov N.V., Vivulanets E.V., Krivoshlyk V.V. 2010. New methods for studying platelets and red blood cells, based upon the low-angle light scattering technique. In: Hemorheology: Blood Flow, Disturbance and Prognosis. Ed. F. Columbus, N.Y.: Nova Sci. Publ., p. 87–124.
Skverchynskaya E.A., Nikitina E.R., Sudnitsyna J.S., Krivchenko A.I., Gambaryan, S.P., Mindukshev I.V. 2015. Hemolysis of erythrocytes or formation of microparticles in apoptosis under oxidative stress? In: Receptors and intracellular signaling (Rus). 2, 564–569. https:// elibrary.ru/item.asp?id=28974843, https://elibrary.ru/ item.asp?id=29279171.
Spring F.A., Gardner B., Anstee D.J. 1992. Evidence that the antigens of the Yt blood group system are located on human erythrocyte acetylcholinesterase. Blood. 80 (8), 2136–2141.
Wessler I., Kirkpatrick C.J., Racké K. 1999. The cholinergic ‘pitfall’: Acetylcholine, a universal cell molecule in biological systems, including humans. Clin. Exp. Pharmacol. Physiol. 26 (3), 198–205.
Wessler I., Kilbinger H., Bittinger F., Unger R., Kirkpatrick C.J. 2003. The non-neuronal cholinergic system in humans: Expression, function and pathophysiology. Life Sci. 72 (18–19), 2055–2061.
Carvalho F.A., Mesquita R., Martins-Silva J., Saldanha C. 2004. Acetylcholine and choline effects on erythrocyte nitrite and nitrate levels. J. App.l Toxicol. 24 (6), 419–427.
Carvalho F.A., Almeida J.P., Fernandes I.O., Freitas-Santos T., Saldanha C. 2008. Non-neuronal cholinergic system and signal transduction pathways mediated by band 3 in red blood cells. Clin. Hemorheol. Microcirc. 40 (3), 207–227.
Teixeira P., Duro N., Napoleão P., Saldanha C. 2015. Acetylcholinesterase conformational states influence nitric oxide mobilization in the erythrocyte. J. Membr. Biol. 248 (2), 349–354.
Prall Y.G., Gambhir K.K., Ampy F.R. 1998. Acetylcholinesterase: An enzymatic marker of human red blood cell aging. Life Sci. 63 (3), 177–184.
Shmurak, V.I., Kurdyukov, I.D., Nadeev A.D., Voitenko N.G., Glaskina L.M., Goncharov N.V. 2012. Biochemical markers of intoxication with organophosphorus toxic substances. Toksikol. vestnik (Rus.). 4, 30–34.
Hundekari I.A., Suryakar A.N., Rathi D.B. 2013. Acute organophosphorus pesticide poisoning in North Karnataka, India: Oxidative damage, haemoglobin level and total leukocyte. Afr. Health Sci. 13 (1), 129–136.
Mindukshev I.V., Krivoshlyk V.V., Ermolaeva E.E., Dobrylko I.A., Senchenkov E.V., Goncharov N.V., Jenkins R.O., Krivchenko A.I. 2007. Necrotic and apoptotic volume changes of erythrocytes investigated by low-angle light scattering technique. Spectroscopy Int. J. 21 (2), 105–120.
Zipser Y., Piade A., Barbul A., Korenstein R., Kosower N.S. 2002. Ca2+ promotes erythrocyte band 3 tyrosine phosphorylation via dissociation of phosphotyrosine phosphatase from band 3. Biochem. J. 368 (1), 137–144.
Shaik N., Lupescu A., Lang F. 2012. Sunitinib-sensitive suicidal erythrocyte death. Cell Physiol. Biochem. 30 (3), 512–522.
Bogdanova A., Makhro A., Wang J., Lipp P., Kaestner L. 2013. Calcium in red blood cells–a perilous balance. Int. J. Mol. Sci. 14 (5), 9848–9872.
ACKNOWLEDGMENTS
Experiments on the Navios flow cytometer have been conducted in the SUC of the IEPB RAS. The work was supported by the Russian Science Foundation (project no. 16-15-00199).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by E. Puchkov
Rights and permissions
About this article
Cite this article
Mindukshev, I.V., Skverchinskaya, E.A., Khmelevskoy, D.A. et al. Acetylcholinesterase Inhibitor Paraoxon Intensifies Oxidative Stress Induced in Rat Erythrocytes In Vitro. Biochem. Moscow Suppl. Ser. A 13, 85–91 (2019). https://doi.org/10.1134/S1990747819010070
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747819010070