Abstract
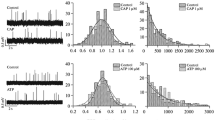
Changes in parameters of spontaneous acetylcholine (ACh) quantal secretion caused by prolonged high-frequency burst activity of neuromuscular junctions and possible involvement of endogenous calcitonin gene-related peptide (CGRP) and its receptors in these changes were studied. With this purpose, miniature endplate potentials (MEPPs) were recorded using standard microelectrode technique in isolated neuromuscular preparations of m. EDL–n. peroneus after a prolonged high-frequency nerve stimulation (30 Hz for 2 min). An increase in the MEPP amplitudes and time course was observed in the postactivation period that reached maximum 20–30 min after nerve stimulation and progressively faded in the following 30 min of recording. Inhibition of vesicular ACh transporter with vesamicol (1 μM) fully prevented this “wave” of the MEPP enhancement. This indicates the presynaptic origin of the MEPP amplitude increase, possibly mediated via intensification of synaptic vesicle loading with ACh and subsequent increase of the quantal size. Competitive antagonist of the CGRP receptor, truncated peptide isoform CGRP8–37 (1 μM), had no effect on spontaneous secretion parameters by itself but was able to prevent the appearance of enhanced MEPPs in the postactivation period. This suggests the involvement of endogenous CGRP and its receptors in the observed MEPP enhancement after an intensive nerve stimulation. Ryanodine in high concentration (1 μM) that blocks ryanodine receptors and stored calcium release did not influence spontaneous ACh secretion but prevented the increase of the MEPP parameters in the postactivation period. Altogether, the data indicate that an intensive nerve stimulation, which activates neuromuscular junctions and muscle contractions, leads to a release of endogenous CGRP into synaptic cleft and this release strongly depends on the efflux of stored calcium. The released endogenous CGRP is able to exert an acute presynaptic effect on nerve terminals, which involves its specific receptor action and intracellular cascades leading to intensification of ACh loading into synaptic vesicles and an increase in the ACh quantal size.
Similar content being viewed by others
References
Van Rossum D., Hanisch U. 1997. Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neurosci. Biobehav. Rev. 21, 649–678.
Csillik B., Tajti L., Kovács T., Kukla E. 1993. Distribution of calcitonin gene-related peptide in vertebrate neuromuscular junctions: Relationship to the acetylcholine receptor. J. Histochem. Cytochem. 41, 1547–1555.
Fernandez H., Chen M., Nadelhaft I., Durr J. 2003. Calcitonin gene-related peptides: Their binding sites and receptor accessory proteins in adult mammalian skeletal muscles. Neuroscience. 119, 335–345.
Matteoli M., Haimann C., Torri-Tarelli F., Polak J., Ceccarelli B., De Camilli P. 1988. Differential effect of alpha-latrotoxin on exocytosis from small synaptic vesicles and from large dense-core vesicles containing calcitonin gene-related peptide at the frog neuromuscular junction. Proc. Natl. Acad. Sci. USA. 85, 7366–7370.
Sakaguchi M., Inaishi Y., Kashihara Y., Kuno M. 1991. Release of calcitonin gene-related peptide from nerve terminals in rat skeletal muscle. J. Physiol. 434, 257–270.
Uchida S., Yamamoto H., Iio S., Matsumoto N., Wang X., Yonehara N., Ima, Y., Inoki R., Yoshida H. 1990. Release of calcitonin gene-related peptide-like immunoreactive substance from neuromuscular junction by nerve excitation and its action on striated muscle. J. Neurochem. 54, 1000–1003.
Lundberg J., Franco-Cereceda A., Alving K., Delay-Goyet P., Lou Y. 1992. Release of calcitonin generelated peptide from sensory neurons. Annu. N. Y. Acad. Sci. 30, 187–193.
Changeux J., Duclert A., Sekine S. 1992. Calcitonin gene-related peptides and neuromuscular interactions. Ann. N. Y. Acad. Sci. 657, 361–378.
Kimura I., Salim S., Dezaki K., Tsuneki H., Abdel-Zaher A. 1998. Calcitonin gene-related peptide potentiates nicotinic acetylcholine receptor-operated slow Ca2+ mobilization at mouse muscle endplates. Br. J. Pharmacol. 125, 277–282.
Di Angelantonio S., Giniatullin R., Costa V., Sokolova E., Nistri A. 2003. Modulation of neuronal nicotinic receptor function by the neuropeptides CGRP and substance P on autonomic nerve cells. Br. J. Pharmacol. 139, 1061–1073.
Fontaine B., Klarsfeld A., Hökfelt T., Changeux J. 1986. Calcitonin gene-related peptide, a peptide present in spinal cord motoneurons, increases the number of acetylcholine receptors in primary cultures of chick embryo myotubes. Neurosci. Lett. 71, 59–65.
da Costa V., Lapa A., Godinho R. 2001. Short-and long-term influences of calcitonin gene-related peptide on the synthesis of acetylcholinesterase in mammalian myotubes. Br. J. Pharmacol. 133, 229–236.
Fernandez H., Ross G., Nadelhaft I. 1999. Neurogenic calcitonin gene-related peptide: A neurotrophic factor in the maintenance of acetylcholinesterase molecular forms in adult skeletal muscles. Brain Res. 844, 83–97.
Rossi S., Dickerson I., Rotundo R. 2003. Localization of the calcitonin gene-related peptide receptor complex at the vertebrate neuromuscular junction and its role in regulating acetylcholinesterase expression. J. Biol. Chem. 278 (27), 24994–5000.
Kimura I., Okazaki M., Nojima H. 1997. Mutual dependence of calcitonin-gene related peptide and acetylcholine release in neuromuscular preparations. Eur. J. Pharmacol. 330, 123–128.
Gaydukov A., Bogacheva P., Balezina O. 2016. Calcitonin gene-related peptide increases acetylcholine quantal size in neuromuscular junctions of mice. Neurosci. Lett. 628, 17–23.
Naves L., Van der Kloot W. 2001. Repetitive nerve stimulation decreases the acetylcholine content of quanta at the frog neuromuscular junction. J. Physiol. 532, 637–647.
Van der Kloot W. 2003. Loading and recycling of synaptic vesicles in the Torpedo electric organ and the vertebrate neuromuscular junction. Prog. Neurobiol. 71, 269–303.
Hodges-Savola C., Fernandez H. 1995. A role for calcitonin gene-related peptide in the regulation of rat skeletal muscle G4 acetylcholinesterase. Neurosci. Lett. 190, 117–120.
Zupanc G. 1996. Peptidergic transmission: From morphological correlates to functional implications. Micron. 27, 35–91.
Shakiryanova D., Tully A., Hewes R., Deitcher D., Levitan E. 2005. Activity-dependent liberation of synaptic neuropeptide vesicles. Nat. Neurosci. 8, 173–178.
Shakiryanova D., Klose M., Zhou Y., Gu T., Deitcher D., Atwood H., Hewes R., Levitan E. 2007. Presynaptic ryanodine receptor-activated calmodulin kinase II increases vesicle mobility and potentiates neuropeptide release. J. Neurosci. 27, 7799–7806.
Nagasaki K., Fleischer S. 1988. Ryanodine sensitivity of the calcium release channel of sarcoplasmic reticulum. Cell Calcium. 9, 1–7.
Van der Kloot W. 1991. The regulation of quantal size. Prog. Neurobiol. 36, 93–130.
Gracz L., Wang W., Parsons S. 1988. Cholinergic synaptic vesicle heterogeneity: Evidence for regulation of acetylcholine transport. Biochemistry. 27, 5268–5274.
Searl T., Prior C., Marshall I. 1991. Acetylcholine recycling and release at rat motor nerve terminals studied using (-)-vesamicol and troxpyrrolium. J. Physiol. 444, 99–116.
Van der Kloot W., Colasante C., Cameron R., Molgó J. 2000. Recycling and refilling of transmitter quanta at the frog neuromuscular junction. J. Physiol. 523 (1), 247–258.
Wong M. Y., Shakiryanova D., Levitan E. S. 2009. Presynaptic ryanodine receptor-CamKII signaling is required for activity-dependent capture of transiting vesicles. J. Mol. Neurosci. 37 (2), 146–150.
Wong M. Y., Cavolo S. L., Levitan E. S. 2015. Synaptic neuropeptide release by dynamin-dependent partial release from circulating vesicles. Mol. Biol. Cell. 26 (13), 2466–2474.
Russell F., King R., Smillie S., Kodji X., Brain S. 2014. Calcitonin gene-related peptide: Physiology and pathophysiology. Physiol. Rev. 94, 1099–1142.
Scalettar B. 2006. How neurosecretory vesicles release their cargo. Neurosci. 12, 164–176.
Levitan E. 2008. Signaling for vesicle mobilization and synaptic plasticity. Mol. Neurobiol. 37, 39–43.
Scuka M., Mozrzymas J. W. 1992. Postsynaptic potentiation and desensitization at the vertebrate end-plate receptors. Prog. Neurobiol. 38 (1), 19–33.
Van der Kloot W., Molgó J. 1994. Quantal acetylcholine release at the vertebrate neuromuscular junction. Physiol. Rev. 74, 899–991.
Andreose J., Fumagalli G., Clementi F. 1994. On the effect of ageing on the distribution of vasoactive intestinal polypeptide and calcitonin gene-related peptide in the rat brain. Neurosci. Lett. 171, 167–171.
Li J., Dahlström A., Kling-Petersen A. 1992. Influence of spinal cord transection on the presence and axonal transport of CGRP-, chromogranin A-, VIP-, synapsin I-, and synaptophysin-like immunoreactivities in rat motor nerve. J. Neurobiol. 23, 1094–1110.
Sala C., Andreose J., Fumagalli G., Lømo T. 1995. Calcitonin gene-related peptide: Possible role in formation and maintenance of neuromuscular junctions. J. Neurosci. 15, 520–528.
Van der Kloot W., Benjamin W., Balezina O. 1998. Calcitonin gene-related peptide acts presynaptically to increase quantal size and output at frog neuromuscular junctions. J. Physiol. 507 (3), 689–695.
Macdonald W., Nielsen O., Clausen T. 2008. Effects of calcitonin gene-related peptide on rat soleus muscle excitability: Mechanisms and physiological significance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295 (4), 1214–1223.
Lu B., Fu W. M. 1995. Regulation of postsynaptic responses by calcitonin gene related peptide and ATP at developing neuromuscular junctions. Can. J. Physiol. Pharmacol. 73 (7), 1050–1056.
Lu B., Fu W. M., Greengard P., Poo M. M. 1993. Calcitonin gene-related peptide potentiates synaptic responses at developing neuromuscular junction. Nature. 363 (6424), 76–79.
Fong S., McLennan I., McIntyre A., Rei, J., Shennan K., Bewick G. 2010. TGF-beta2 alters the characteristics of the neuromuscular junction by regulating presynaptic quantal size. Proc. Natl. Acad. Sci. USA. 107, 13515–13519.
Melo C., Mele M., Curcio M., Comprido D., Silva C, Duarte C. 2013. BDNF regulates the expression and distribution of vesicular glutamate transporters in cultured hippocampal neurons. PLoS One. 8, e53793.
Van der Kloot W., Brănişteanu D. 1992. Effects of activators and inhibitors of protein kinase A on increases in quantal size at the frog neuromuscular junction. Pflugers. Arch. 420, 336–341.
Hoffmann C., Weigert C. 2017. Skeletal muscle as an endocrine organ: The role of myokines in exercise adaptations. Cold Spring Harb. Perspect. Med. 7 (11), a029793.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © P.O. Bogacheva, E.A. Golikova, O.P. Balezina, 2018, published in Biologicheskie Membrany, 2018, Vol. 35, No. 4, pp. 297–308.
Rights and permissions
About this article
Cite this article
Bogacheva, P.O., Golikova, E.A. & Balezina, O.P. The Role of Endogenous Calcitonin Gene-Related Peptide in the Neurotransmitter Quantal Size Increase in Mouse Neuromuscular Junctions. Biochem. Moscow Suppl. Ser. A 12, 268–277 (2018). https://doi.org/10.1134/S1990747818030029
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747818030029