Abstract
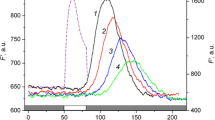
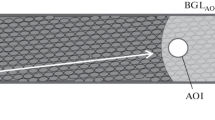
Cytoplasmic streaming is vital for plant cells; however, its relation to cell functions remains largely undisclosed. Microfluorometry of chloroplasts in vivo and measurements of cell surface pH under localized illumination of cell regions located upstream the cytoplasmic flow, at a distance of few millimeters from the analyzed area, is a new means to reveal the role of liquid flow for signal transmission in large cells, such as internodes of characean algae. Properties of photoinduced signals transmitted along the cell can be clarified by comparing the effects of pointed illumination under conditions of continuous and briefly arrested cytoplasmic flow. Chlorophyll fluorescence measurements with the use of saturation pulse method showed that excitation-induced cessation of cytoplasmic streaming, concomitant with the period of localized illumination, caused a significant delay and deceleration of the lateral transmission of the photoinduced signal and, in addition, diminished the peak of maximal fluorescence F m′ in the cell response to propagated signals. The relative extent of the peak suppression was small in cell regions producing light-dependent external alkaline zones and increased substantially for cell regions with slightly acidic external pH. These and other results indicate the possible role of cytoplasmic pH in controlling chlorophyll fluorescence and photosynthetic activity in vivo. When the period of streaming cessation coincided with localized illumination, the velocity of cytoplasmic flow recovered slower than after arrest of the flow without additional illumination. The results are promising for further analysis of regulatory and protective functions of cytoplasmic streaming in photosynthesizing plant cells.
Similar content being viewed by others
References
Pickard W.F. 2003. The role of cytoplasmic streaming in symplastic transport. Plant, Cell Environ. 26, 1–15.
Verchot-Lubicz J., Goldstein R.E. 2010. Cytoplasmic streaming enables the distribution of molecules and vesicles in large plant cells. Protoplasma. 240, 99–107.
Goldstein R.E., Tuval I., van de Meent J.W. 2008. Microfluidics of cytoplasmic streaming and its implications for intracellular transport. Proc. Natl. Acad. Sci. USA. 105, 3663–3667.
Bulychev A.A., Dodonova S.O. 2011. Effects of cyclosis on chloroplast-cytoplasm interactions revealed with localized lighting in Characean cells at rest and after electrical excitation. Biochim. Biophys. Acta. 1807, 1221–1230.
Dodonova S.O., Bulychev A.A. 2011. Cyclosis-related asymmetry of chloroplast-plasma membrane interactions at the margins of illuminated area in Chara corallina cells. Protoplasma. 248, 737–749.
Eremin A., Bulychev A.A., Hauser M.J.B. 2013. Cyclosis-mediated transfer of H2O2 elicited by localized illumination of Chara cells and its relevance to the formation of pH bands. Protoplasma. 250, 1339–1349.
Bulychev A.A., Alova A.V., Rubin A.B. 2013. Propagation of photoinduced signals with the cytoplasmic flow along Characean internodes: Evidence from changes in chloroplast fluorescence and surface pH. Eur. Biophys. J. 42, 441–453.
Bulychev A.A., Alova A.V., Rubin A.B. 2013. Fluorescence transients in chloroplasts of Chara corallina cells during transmission of photoinduced signal with the streaming cytoplasm. Russ. J. Plant Physiol. 60, 33–40.
Harada A., Shimazaki K. 2009. Measurement of changes in cytosolic Ca2+ in Arabidopsis guard cells and mesophyll cells in response to blue light. Plant Cell Physiol. 50, 360–373.
Miller A.J., Sanders D. 1987. Depletion of cytosolic free calcium induced by photosynthesis. Nature. 326, 397–400.
Remiš D., Bulychev A.A., Kurella G.A. 1988. Photoinduced pH changes in the vicinity of isolated Peperomia metallica chloroplasts. J. Exp. Bot. 39, 633–640.
Naydov I.A., Mubarakshina M.M., Ivanov B.N. 2012. Formation kinetics and H2O2 distribution in chloroplasts and protoplasts of photosynthetic leaf cells of higher plants under illumination. Biochemistry (Moscow). 77, 143–151.
Felle H., Bertl A. 1986. Light-induced cytoplasmic pH changes and their interrelation to the activity of the electrogenic proton pump in Riccia fluitans. Biochim. Biophys. Acta. 848, 176–182.
Kamiya N. 1959. Protoplasmic streaming. Wien: Springer.
Williamson R.E., Ashley C.C. 1982. Free Ca2+ and cytoplasmic streaming in the alga Chara. Nature. 296, 647–651.
Tominaga Y., Shimmen T., Tazawa M. 1983. Control of cytoplasmic streaming by extracellular Ca2+ in permeabilized Nitella cells. Protoplasma. 116, 75–77.
Yokota E., Muto S., Shimmen T. 1999. Inhibitory regulation of higher-plant myosin by Ca2+ ions. Plant Physiol. 119, 231–239.
Shimmen T. 2007. The sliding theory of cytoplasmic streaming: Fifty years of progress. J. Plant Res. 120, 31–43.
Awata J., Saitoh K., Shimada K., Kashiyama T., Yamamoto K. 2001. Effects of Ca2+ and calmodulin on the motile activity of characean myosin in vitro. Plant Cell Physiol. 42, 828–834.
Tsuchiya Y., Yamazaki H., Aoki T. 1991. Steady and transient behaviors of protoplasmic streaming in Nitella internodal cell. Biophys. J. 59, 249–251.
Bulychev A.A., Kamzolkina N.A. 2006. Differential effects of plasma membrane electric excitation on H+ fluxes and photosynthesis in characean cells. Bioelectrochemistry. 69, 209–215.
Bulychev A.A., Kamzolkina N.A. 2006. Effect of action potential on photosynthesis and spatially distributed H+ fluxes in cells and chloroplasts of Chara corallina. Russ. J. Plant Physiol. 53, 1–9.
Bulychev A.A., Kamzolkina N.A., Luengviriya J., Rubin A.B., Mueller S.C. 2004. Effect of a single excitation stimulus on photosynthetic activity and lightdependent pH banding in Chara cells. J. Membr. Biol. 202, 11–19.
Krupenina N.A., Bulychev A.A. 2007. Action potential in a plant cell lowers the light requirement for non-photochemical energy-dependent quenching of chlorophyll fluorescence. Biochim. Biophys. Acta. 1767, 781–788.
Bulychev A.A. 2012. Membrane excitation and cytoplasmic streaming as modulators of photosynthesis and proton flows in Characean cells. In: Plant electrophysiology: Methods and cell electrophysiology. Ed. Volkov A.G. Berlin: Springer, p. 273–300.
van de Meent J.W., Sederman A.J., Gladden L.F., Goldstein R.E. 2010. Measurement of cytoplasmic streaming in single plant cells by magnetic resonance velocimetry. J. Fluid Mech. 642, 5–14.
Krupenina N.A., Bulychev A.A., Roelfsema M.B.G., Schreiber U. 2008. Action potential in Chara cells intensifies spatial patterns of photosynthetic electron flow and non-photochemical quenching in parallel with inhibition of pH banding. Photochem. Photobiol. Sci. 7, 681–688.
Bulychev A.A., Krupenina N.A. 2009. Transient removal of alkaline zones after excitation of Chara cells is associated with inactivation of high conductance in the plasmalemma. Plant Signal. Behav. 4, 24–31.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.A. Bulychev, A.V. Komarova, 2014, published in Biologicheskie Membrany, 2014, Vol. 31, No. 5, pp. 352–363.
Rights and permissions
About this article
Cite this article
Bulychev, A.A., Komarova, A.V. Lateral transport of photosynthetically active intermediate at rest and after excitation of Chara cells. Biochem. Moscow Suppl. Ser. A 8, 314–323 (2014). https://doi.org/10.1134/S199074781405002X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S199074781405002X