Abstract
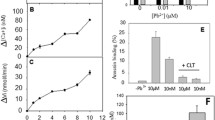
Lipid bodies (LB) are dynamic inducible organelles with the key roles in cellular lipid metabolism, intracellular trafficking and signaling. These structures have a neutral lipid-rich core which contains mainly triacylglycerides (TAG) and cholesterol esters (CE). With the use of flow cytometry and lipophylic fluorescent dye Nile red (NR) we studied LB biogenesis in nonfixed freshly isolated epithelial cells derived from the frog (Rana temporaria L.) urinary bladder. These cells are characterized by numerous small LB located diffusely in the cytoplasm. To target neutral lipids in LB, we used arachidonic acid (AA), an inducer of LB biogenesis in different cell types, and methyl-β-cyclodextrin (MβCD), non-permeable cholesterol acceptor, widely used to extract cholesterol from the lipid rafts. The cells were incubated with 10–50 μM AA for 1 h or with 400–2000 μM MβCD for 30 min; after that they were stained with NR, and fluorescence was measured by flow cytometer at λex = 488 nm and λem = 575 ± 15 nm. In parallel, side scatter (SS) was analyzed. It was found that AA in a dose-dependent manner increased NR fluorescence. At a maximal concentration used, AA increased NR fluorescence and SS by 41 ± 2% and by 15 ± 3% (p < 0.001), respectively. Analysis of lipid composition of cell extracts revealed a significant increase of TAG by 10 μM AA. MβCD starting from 400 μM decreased dose-dependently the NR fluorescence and SS. Its effect was accompanied by a decrease of cellular free cholesterol by 6% (p < 0.01) and cholesterol ester by 21% (p < 0.001). This fact indicates mobilization of cholesterol from cholesterol esters stored in LB in order to restore cholesterol level in the plasma membrane. Taken together, our data demonstrate that flow cytometry in combination with NR staining represents a reliable tool to be used for recording of changes in different neutral lipids class content within LB in living cells non-specialized on the fat storage.
Similar content being viewed by others
References
Fujimoto T., Ohsaki Y., Cheng J., Suzuki M., Shinohara Y. 2008. Lipid droplets: A classic organelle with new outfits. Histochem. Cell. Biol. 130(2), 263–279.
Ohsaki Y., Cheng J., Suzuki M., Shinohara Y., Fujita A., Fujimoto T. 2008. Biogenesis of cytoplasmic lipid droplets: From the lipid ester globule in the membrane to the visible structure. Biochim. Biophys. Acta. 1791, 399–407.
Greenberg A., Egan J., Wek S.A., Garty N., Blanchette-Mackie E., Londos C. 1991. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J. Biol. Chem. 266(17), 11341–11346.
Wolins N., Rubin B., Brasaemle D. 2001. TIP47 associates with lipid droplets. J. Biol. Chem. 276, 5101–5108.
Londos C., Sztalryd C., Tansey J., Kimmel A. 2005. Role of PAT proteins in lipid metabolism. Biochimie. 87(1), 45–49.
Brasaemle D. 2007. Thematic review series: Adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J. Lipid Res. 48(12), 2547–2559.
Johnson M., Vaughn B., Triggiani M., Swan D., Fonteh A., Chilton F. 1999. Role of arachidonyl triglycerides within lipid bodies in eicosanoid formation by human polymorphonuclear cells. Am. J. Respire Cell. Mol. Biol. 21, 253–258.
Farese R., Walther T. 2009. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell. 139, 855–860.
Olofsson S., Bostrom P., Andersson L. et al., 2009. Lipid droplets as dynamic organelles connecting storage and efflux of lipids. Biochim. Biophys. Acta. 1791(6), 448–458.
Walther T., Farese R. 2009. The life of lipid droplets. Biochim. Biophys. Acta. 1791(6), 459–466.
Roingeard P., Hourioux C. 2008. Hepatitis C virus core protein, lipid droplets and steatosis. J. Virus Hepatitis. 15, 157–164.
Bozza P.T., Yu W., Penrose J.F., Morgan E.S., Dvorak A.M., Weller P.F. 1997. Eosinophils lipid bodies: Specific, inducible intracellular sites for enhanced eicosanoid formation. J. Exp. Med. 186, 909–920.
Bozza P., Viola J. 2010. Lipid droplets in inflammation and cancer. Prostaglandins, Leucotrienes and Essential Fatty Acids. 82, 243–250.
Dvorak A.M., Morgan E., Tzizik D.M., Weller P.F. 1994. Prostaglandin endoperoxide synthase (cyclooxygenase): Ultrastructural localization to non-membrane-bound cytoplasmic lipid bodies in human eosinophils and murine 3T3 fibroblasts. Int. Arch. Allergy Immunol. 105, 245–250.
Maya-Monteiro C.M., Almeida P.E., D’Avila H., Martins A.S., Rezende A.P., Castro-Faria-Neto H., Bozza P.T. 2008. Leptin induces macrophage lipid body formation by a phosphatidylinositol 3-kinase- and mammalian target of rapamycin-dependent mechanism. J. Biol. Chem. 283(4), 2203–2210.
Weller P.F., Ryeom S.W., Picard S.T., Ackerman S.J., Dvorak A.M. 1991. Cytoplasmic lipid bodies of neutrophils: Formation induced by cis-unsaturated fatty acids and mediated by protein kinase C. J. Cell Biol. 113(1), 137–146.
Moreira L.S., Piva B., Gentile L.B., Mesquita-Santos F.P., D’Avila H., Maya-Monteiro C.M., Bozza P.T., Bandeira-Melo C., Diaz B.L. 2009. Cytosolic phospholipase A2-driven PGE2 synthesis within unsaturated fatty acids-induced lipid bodies of epithelial cells. Biochim. Biophys. Acta. 1791(3), 156–165.
Bozaquel-Morais B., Madeira J., Maya-Monteiro C., Masuda C., Montero-Lomeli M. 2010. A new fluorescence-based method identifies protein phosphatases regulating lipid droplet metabolism. PLoS One. 28, 5 (10), doi: 10.1371/journal.pone.0013692.
Greenspan P., Mayer E.P., Fowler S.D. 1985. Nile red: A selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 100(3), 965–973.
Schaedlich K., Knelangen J.M, Navarrete Santos A., Fischer B., Navarrete Santos A. 2010. A simple method to sort ESC-derived adipocytes. Cytometry A. 77(10), 990–995.
Hassall D.G. 1992. Three probe flow cytometry of a human foam-cell forming macrophage. Cytometry. 13(4), 381–388.
da Silva T.L., Feijão D., Reis A. 2010. Using multiparameter flow cytometry to monitor the yeast Rhodotorula glutinis CCMI 145 batch growth and oil production towards biodiesel. Appl. Biochem. Biotechnol. 162(8), 2166–2176.
Raschke D., Knorr D. 2009. Rapid monitoring of cell size, vitality and lipid droplet development in the oleaginous yeast Waltomyces lipofer. J. Microbiol. Methods. 79(2), 178–183.
Cirulis J.T., Strasser B.C., Scott J.A., Ros G.M. 2012. Optimization of staining conditions for microalgae with three lipophilic dyes to reduce precipitation and fluorescence variability. Cytometry A. 81, 618–626.
Greenspan P., Fowler S.D. 1985. Spectrofluorometric studies of the lipid probe, nile red. J. Lipid Res. 26(7), 781–789.
Triggiani M., Oriente A., de Crescenzo G., Rossi G., Marone G. 1995. Biochemical functions of a pool of arachidonic acid associated with triglycerides in human inflammatory cells. Int. Arch. Allergy Immunol. 107(1–3), 261–263.
Dichlberger A., Schlager S., Lappalainen J., Käkelä R., Hattula K., Butcher S.J., Schneider W.J., Kovanen P.T. 2011. Lipid body formation during maturation of human mast cells. J. Lipid Res. 52(12), 2198–2208.
Zidovetzki R., Levitan I. 2007. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim. Biophys. Acta. 1768(6), 1311–1124.
Chang T., Chang C., Ohgami N., Yamauchi Y. 2006. Cholesterol sensing, trafficking, and esterification. Annu. Rev. Cell Dev. Biol. 22, 129–157.
Mahammad S., Parmryd I. 2008. Cholesterol homeostasis in T cells. Methyl-beta-cyclodextrin treatment results in equal loss of cholesterol from Triton X-100 soluble and insoluble fractions. Biochim. Biophys. Acta. 1778(5), 1251–1258.
Folch J., Lees M., Sloane-Stanley G. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226, 497–509.
Gubern A., Casas J., Barceló-Torns M., Barneda D., de la Rosa X., Masgrau R., Picatoste F., Balsinde J., Balboa M.A., Claro E. 2008. Group IVA phospholipase A2 is necessary for the biogenesis of lipid droplets. J. Biol. Chem. 283(41), 27369–27382.
Diaz G., Melis M., Batetta B., Angius F., Falchi A. 2008. Hydrophobic characterization of intracellular lipids in situ by Nile Red red/yellow emission ratio. Micron. 39(7), 819–824.
Romek M., Gajda B., Krzysztofowicz E., Kepczynski M., Smorag Z. 2011. New technique to quantify the lipid composition of lipid droplets in porcine oocytes and pre-implantation embryos using Nile Red fluorescent probe. Theriogenology. 75(1), 42–54.
Neufeld E.B., Cooney A.M., Pitha J., Dawidowicz E.A., Dwyer N.K., Pentchev P.G., Blanchette-Mackie E.J. 1996. Intracellular trafficking of cholesterol monitored with a cyclodextrin. J. Biol. Chem. 271, 21604–21613.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.M. Fock, E.V. Fedorova, R.G. Parnova, 2013, published in Biologicheskie Membrany, 2013, Vol. 30, No. 3, pp. 221–229.
Rights and permissions
About this article
Cite this article
Fock, E.M., Fedorova, E.V. & Parnova, R.G. Application of flow cytometry for estimation of lipid content changes induced by arachidonic acid and methyl-β-cyclodextrin in the lipid bodies of epithelial cells. Biochem. Moscow Suppl. Ser. A 7, 134–140 (2013). https://doi.org/10.1134/S1990747813020049
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747813020049