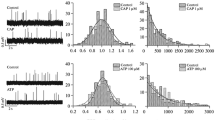
Abstract
A number of different types of presynaptic receptors was revealed in central and peripheral chemical synapses activated both by main mediator and co-mediators released simultaneously. Physiological significance and mechanisms of functioning of these receptors are not clear yet. They are assumed to provide negative or positive feedback decreasing or increasing the number of neurotransmitter quanta released in response to nerve impulse and thus regulating synaptic transmission. At the same time, there is one more way of secretion process modulation associated with the changes of timing of transmitter release. This mechanism was shown to contribute to the efficiency of synaptic transmission. The role of presynaptic receptors in regulation of the kinetics of quanta release is one of the interesting questions of modern neurophysiology. This paper overviews the results obtained by the authors that demonstrate the contribution of presynaptic receptors of different types into the regulation of temporal parameters of quantal secretion at the vertebrates neuromuscular junction. It was shown that activation of the cholinergic nicotinic receptors leads to a decrease of the amplitude of postsynaptic response not only due to reduction of the quantity of released quanta but also due to increased the level of asynchronous release. On the contrary, the facilitating effect of catecholamines on the neuromuscular synapse is the result of activation of presynaptic β1-adrenoreceptors which leads to greater synchronization of release process and, consequently, to the increase of the amplitude of the postsynaptic response. Presynaptic purine receptors, involved in the modulation the intensity of secretion, are also capable of alteration of the time course of secretion. Activation of ryanodine receptors results in the increase of the number of quanta released with prolonged latencies leading to appearance of the phase of delayed asynchronous neurotransmitter release.
Similar content being viewed by others
References
Langer S.Z. 2008. Presynaptic autoreceptors regulating transmitter release. Neurochem. Int. 52(1–2), 26–30.
Miller R.J. 1998. Presynaptic receptors. Annu. Rev. Pharmacol. Toxicol. 38, 201–207.
Burnstock G. 2007. Purine and pyrimidine receptors. Cell. Mol. Life Sci. 64, 1471–1483.
Liu Q., Chen B., Yankova M., Morest D., Maryon E., Hand A., Nonet M., Wang Z-W. 2005. Presynaptic ryanodine receptors are required for normal quantal size at the Caenorhabditis elegans neuromuscular junction. J. Neurosci. 25(29), 6745–6754.
Van der Kloot W., Molgo J. 1994. Quantal acetylcholine release at the vertebrate neuromuscular junction. Physiol. Rev. 74(4), 899–991.
Bowman W., Prior C., Marshall I. 1990. Presynaptic receptors in the neuromuscular junction. Ann. NY. Acad. Sci. 604, 69–81.
Starke K. 1989. Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol. Rev. 69, 864–989.
Parnas H., Segel L., Dudel J., Parnas I. 2000. Autoreceptors, membrane potential and regulation the transmitter release. Trends Neurosci. 23, 60–68.
Miyamoto M. 1977. The actions of cholinergic drugs on motor nerve terminals. Pharmacol. Rev. 29(3), 221–247.
Ciani S., Edwards C. 1963. The effect of acetylcholine on neuromuscular transmission in the frog. J. Pharmacol. Exp. Therap. 142, 21–23.
Silinsky E.M., Redman R.S. 1996. Synchronous release of ATP and neurotransmitter within milliseconds of a motor nerve impulse in the frog. J. Physiol. 492, 815–822.
Giniatullin R.A., Sokolova E.M. 1998. ATP and adenosine inhibit transmitter release at the frog neuromuscular junction through distinct presynaptic receptors. Br. J. Pharmacol. 124, 839–844.
Wessler J., Anschuetz S. 1988. β-Adrenoreceptor stimulation enhances transmitter output from the rat phrenic nerve. Br. J. Pharmacol. 94(3), 669–674.
Wessler I., Holzer G., Kanstler A. 1990. Stimulation of β1 adrenoreceptors enhances electrically evoked [3H]-acetylcholine release from rat phrenic nerve. Clin. Exp. Pharmacol. Physiol. 17, 23–32.
Slater C. 2008. Reliability of neuromuscular transmission and how it is maintained. Handb. Clin. Neurol. 91, 27–101.
Lin J.-W., Faber S. 2002. Modulation of synaptic delay during synaptic plasticity. Trends Neurosci. 25, 449–455.
Fesce R. 1999. The kinetics of nerve-evoked quantal secretion. Philos. Trans. R. Soc. London. B. Biol. Sci. 354, 319–329.
Katz B., Miledi R. 1965. The measurement of synaptic delay and the time course of acetylcholine release at the neuromuscular junction. Proc. R. Soc. London. B. 161, 483–495.
Barrett E.F., Stevens C.F. 1972. The kinetics of transmitter release at the frog neuromuscular junction. J. Physiol. 227, 691–708.
Sabatini B., Regehr W. 1999. Timing of synaptic transmission. Annu. Rev. Physiol. 61, 521–542.
Soucek B. 1971. Influence of latency fluctuations and the quantal process of transmitter release on the endplate potential’s amplitude distribution. Biophys. J. 11, 127–139.
Giniatullin R., Kheeroug L., Vyskocil F. 1995. Modelling endplate current: dependence on quantum secretion probability and postsynaptic miniature current parameters. Eur. Biophys. J. 23, 443–446.
Gilmanov I., Samigullin D., Vyskocil F., Nikolsky E., Bukharaeva E. 2008. Modeling of quantal neurotransmitter release kinetics in the presence of fixed and mobile calcium buffers. J. Comput. Neurosci. 25, 296–307.
Van der Kloot W. 1988. Estimating the timing of quantal releases during end-plate currents at the frog neuromuscular junction. J. Physiol. 402, 595–603.
Aumann Y., Parnas H. 1991. Evaluation of the time course of neurotransmitter release from the measured psc and mpsc. Bull. Math. Biol. 53, 537–555.
Vorobieva O., Hackett J., Worden M., Bykhovskaia M. 1999. Evaluation of quantal neurosecretion from evoked and miniature postsynaptic responses by deconvolution method. J. Neurosci. Meth. 92, 91–99.
Gainulov R., Bukharaeva E., Nikolsky E. 2002. A method for assessing the kinetics of evoked secretion of transmitter quanta determining the generation of multiquantum endplate currents. Neurosci. Behav. Physiol. 32, 613–616.
Vizi E.S., Somogyi G.T. 1989. Prejunctional modulation of acetylcholine release from the skeletal neuromuscular junction: link between positive (nicotinic)and negative (muscarinic)-feedback modulation. Brit. J. Pharmacol. 97, 65–70.
Nikolsky E., Giniatullin R. 1979. Termination of the presynaptic effect of carbacholine by tubocurarine. Bull. Exper. Biol. Med. 87(2), 171–174.
Katz B., Thesleff S. 1957. A study of the desensitization produced by acetylcholine at the motor end-plate. J. Physiol. 138, 63–80.
Matzner H., Parnas H., Parnas I. 1988. Presynaptic effects of d-tubocurarine on neurotransmitter release at the neuromuscular junction of the frog. J. Physiol. 398, 109–121.
Slutsky I., Parnas H., Parnas I. 1999. Presynaptic effects of muscarine on Ach release at the frog neuromuscular junction. J. Physiol. 514, 769–782.
Slutsky I., Silman I., Parnas I., Parnas H. 2001. Presynaptic M2 muscarinic receptors are involved in controlling the kinetics of Ach release at the frog neuromuscular junction. J. Physiol. 536(3), 717–725.
Santafe M.M., Salon I., Garcia N., Lanuza M.A., Uchitel O.D., Tomas J. 2003. Modulation of Ach release by presynaptic muscarinic auto-receptors in the neuromuscular junction from the newborn to the adult rat. Eur. J. Neurosci. 17, 1–9.
Dudel J. 2007. The time course of transmitter release in mouse motor nerve terminals is differentially affected by activation of muscarinic M1 or M2 receptors. Eur. J. Neurosci. 26, 2160–2168.
Nikolsky E., Samigullin D., Vyskocil F., Bukharaeva E., Magazanik L. 2004. Cholinergic regulation of the quantal release at frog neuromuscular junction. J. Physiol. 560, 77–88.
Bukharaeva E., Nikolsky E. 2010. Changes in the kinetics of quanta secretion — effective mechanism os synaptic transmission modulation. Rossiiskii fiziol. zhurn. (Rus.). 96(8), 766–777.
Kovyazina I., Tsentsevitsky A., Nikolsky E., Bukharaeva E. 2010. Kinetics of acetylcholine quanta release at the neuromuscular junction during high-frequency nerve stimulation. Eur. J. Neurosci. 32, 1480–1489.
Kuba K., Tomita T. 1971. Noradrenaline action on nerve terminal in the rat diaphragm. J. Physiol. 217, 19–31.
Yawo H. 1996. Noradrenaline modulates transmitter release by enhancing the Ca2+ sensitivity of exocytosis in the chick ciliary presynaptic terminal. J. Physiol. 493, 385–391.
Kuba K. 1970. Effects of catecholamines on the neuromuscular junction in the rat diaphragm. J. Physiol. 211, 551–570.
Vizi S. 1991. Evidence that catecholamines increase acetylcholine release from neuromuscular junction through stimulation of alpha-1 adrenoreceptors. Naunyn-Schmiedeberg’s Arch. Pharmacol. 343, 435–438.
Orbeli L.A. 1923. Die sympatetische Innervation der Skelettmuskeln. Bull. Inst. Sci. Leshaft. 6, 194–197.
Melichar I., Brozek G., Jansky L., Vyskocil F. 1973. Effect of hibernation and noradrenaline on acetylcholine release and action at neuromuscular junction of golden hamster (Mesocricetus auratus). Pflugers Arch. 345, 107–122.
Bukharaeva E.A., Nikolsky E.E., Kim K.Kh., Moravec J., Vyskocil F. 1999. Noradrenaline synchronizes evoked quantal release at frog neuromuscular junction. J. Physiol. 517(3), 879–888.
Bukharaeva E.A., Nikolsky E.E., Kim K.Kh., Gainulov R.Kh., Vyskocil F. 1998. Catecholamines change the time course of the evoked quanta mediator release via β-adrenoreceptors. J. Neurochemistry. 71, S49B.
Silinsky E.M. 2004. Adenosine decreases both presynaptic calcium currents and neurotransmitter release at the mouse neuromuscular junction. J. Physiol. 558, 389–401.
Todd K., Robitaille R. 2006. Purinergic modulation of synaptic signaling at the neuromuscular junction. Pflugers Arch.-Eur. J. Physiol. 452, 608–614.
Tsentsevitsky A., Nikolsky E., Giniatullin R., Bukharaeva E. 2011. Opposite modulation of time course of quantal release in two parts of the same synapse by reactive oxygen species. Neuroscience. 189, 93–99.
Rahamimoff R., Yaari Y. 1973. Delayed release of transmitter at the frog neuromuscular junction. J. Physiol. 228, 241–257.
Atluri P., Regehr W. 1998. Delayed release of neurotransmitter from cerebellar granule cells. J. Neurosci. 18, 8214–8227.
Hagler D.J., Goda Y. 2001. Properties of synchronous and asynchronous release during pulse train depression in cultured hippocampal neurons. J. Neurophysiol. 85, 2324–2334.
Yoshihara M., Guan Z., Littleton T. 2010. Differential regulation of synchronous versus asynchronous neurotransmitter release by the C2 domains of synaptotagmin 1. Proc. Natl. Acad. Sci. USA. 107(33), 14869–14874.
Otsu Y., Shahrezaei V., Li B., Raymond L., Delaney K., Murphy T. 2004. Competition between phasic and asynchronous release for recovered synaptic vesicles at developing hippocampal autaptic synapses. J. Neurosci. 24, 420–433.
Hestrin S., Galarreta M. 2005. Synchronous versus asynchronous transmitter release: a tale of two types of inhibitory neurons. Nat. Neurosci. 8, 1283–1284.
Iremonger K.J., Bains J.S. 2007. Integration of asynchronously released quanta prolongs the postsynaptic spike window. J. Neurosci. 27(25), 6684–6691.
Manseau F., Marinelli S., Meńdez P., Schwaller B., Prince D., Huguenard J., Bacci A. 2010. Desynchronization of neocortical networks by asynchronous release of GABA at autaptic and synaptic contacts from fastspiking interneurons. PLoS Biol. 2010 Sep 28;8(9). pii: e1000492.
Lu T., Trussell L. 2000. Inhibitory transmission mediated by asynchronous transmitter release. Neuron. 26, 683–694.
Chen C., Regehr W. 1999. Contributions of residual calcium to fast synaptic transmission. J. Neurosci. 19, 6257–6266.
Balezina O.P. 2002. Role of intracellular calcium stores in nerve endings in regulation of neurotransmission secretion. Usp. Fiziol. Nauk (Rus.). 33(3), 38–56.
Bouchard R., Pattarini R., Geiger J.D. 2003. Presence and functional significance of presynaptic ryanodine receptors. Progr. Neurobiol. 69(6), 391–418.
Narita K., Akita T., Osanai M., Shirasaki T., Kijima H., Kuba K. 1998. A Ca2+-induced Ca2+ release mechanism involved in asynchronous exocytosis at frog motor nerve terminals. J. Gen. Physiol. 112, 593–609.
Narita K., Akita T., Hachisuka J., Huang S.-M., Ochi K., Kuba K. 1989. Functional coupling of Ca2+ channels to ryanodine receptors at presynaptic terminals. J. Gen. Physiol. 115, 519–532.
Bukharaeva E.A., Samigullin D., Nikolsky E.E., Magazanik L.G. 2007. Modulation of the kinetics of evoked quantal release at mouse neuromuscular junctions by calcium and strontium. J. Neurochem. 100, 939–949.
Bukharaeva E., Samigullin D., Nikolsky E., Vyskocil F. 2002. Protein kinase A cascade regulates quantal release dispersion at frog muscle endplate. J. Physiol. 538, 837–848.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © D.V. Samigullin, V.F. Khuzakhmetova, A.N. Tsentsevitsky, E.A. Bukharaeva, 2011, published in Biologicheskie Membrany, 2011, Vol. 28, No. 6, pp. 436–445.
The article was translated by the authors.
Rights and permissions
About this article
Cite this article
Samigullin, D.V., Khuzakhmetova, V.F., Tsentsevitsky, A.N. et al. Presynaptic receptors regulating the time course of neurotransmitter release from vertebrate nerve endings. Biochem. Moscow Suppl. Ser. A 6, 1–8 (2012). https://doi.org/10.1134/S1990747811060134
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747811060134