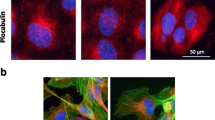
Abstract
The endothelium lining the inner surface of blood vessels fulfils an important barrier function and specifically, it controls vascular membrane permeability as well as nutrient and metabolite exchange in circulating blood and tissue fluids. Disturbances in vascular endothelium barrier function (vascular endothelium dysfunction) are coupled to cytoskeleton rearrangements, actomyosin contractility, and as a consequence, formation of paracellular gaps between endothelial cells. Microtubules constitute the first effector link in the reaction cascade resulting in vascular endothelium dysfunction. Increased vascular permeability associated with many human diseases is also manifested as a side effect in anticancer mitosis-blocking therapy. The aim of this study was to examine the possibility of preventing side effects of mitostatic drugs in patients with vascular endothelium dysfunction and to establish effective doses able to disrupt the microtubular network without interfering with the endothelial barrier function. Previously, it was found that the population of endothelial cell microtubules is heterogeneous. Along with dynamic microtubules, cell cytoplasm contains a certain amount of post-translationally modified microtubules that are less active and less susceptible to external influences than dynamic microtubules. We have shown that the area occupied with stable microtubules is relatively large (approx. one third of the total cell area). We assume that it can account for a higher resistance of the endothelial monolayer to factors responsible for vascular endothelium dysfunction. This hypothesis was validated in this study, in which nocodazole was used to induce vascular endothelium dysfunction in lung endothelial cells. The effect of nocodazole on endothelial cell cytoskeleton was found to be dose-dependent. Nocodazole in micromolar concentrations not only irreversibly changed the barrier function, but also upset the viability of endothelial cells and induced their death. Nanomolar concentrations of nocodazole also increased the permeability of the endothelial monolayer; this effect was reversible at the drug concentration ranging from 100 to 200 nM. At 100 nM, nocodazole induced partial disruption of the microtubule network near the cell margin without any appreciable effect on acetylated microtubules and actin filaments. At 200 nM, nocodazole exerted a pronounced effect on the system of dynamic (but not acetylated) microtubules and increased the population of actin filaments in the central region of the cell. Our data suggest that disruption of peripheral microtubules triggers a cascade of reactions culminating in endothelial barrier dysfunction; however, the existence of a large population of microtubules resistant to nanomolar concentrations of the drug provides higher viability of endothelial cells and restores their functional activity.
Similar content being viewed by others
References
Lum, H. and Malik, A.B., Mechanisms of Increased Endothelial Permeability, Can. J. Physiol. Pharmacol., 1996, vol. 74, pp. 787–800.
Dudek, S.M. and Garcia, J.G., Cyloskeletal Regulation of Pulmonary Vascular Permeability, J. Appl. Physiol., 2001, vol. 91, pp. 1487–1500.
Bogatcheva, N.V., Garcia, J.G.N., and Verin A.D., Molecular Mechanisms of Thrombin-Induced Endothelial Cell Permeability, Biochemistry (Moscow), 2002, vol. 67, no. 1, pp. 75–84.
Birukova, A., Birukov, K., Smurova, K., Kaibuchi, K., Alieva, I., Garcia, J.G., and Verin, A., Novel Role of Microtubules in Thrombin-Induced Endothelial Barrier Dysfunction, FASEB J., 2004, vol. 18, pp. 1879–1890.
Birukova, A., Smurova, K., Birukov, K., Usatyuk, P., Liu, F., Kaibuchi, K., Ricks-Cord, A., Natarajan, V., Alieva, I., Garcia, J.G., and Verin, A., Microtubule Disassembly Induces Cytoskeletal Remodeling and Vascular Barrier Dysfunction: Role of Rho-Dependent Mechanisms, J. Cell. Physiol., 2004, vol. 201, pp. 55–70.
Garcia, J.G., Davis, H.W., and Patterson, C.E., Regulation of Endothelial Cell Gap Formation and Barrier Dysfunction: Role of Myosin Light Chain Phosphorylation, J. Cell. Physiol., 1995, vol. 163, pp. 510–522.
Garcia, J.G., Verin, A.D., and Schaphorst, K.L., Regulation of Thrombin-Mediated Endothelial Cell Contraction and Permeability, Semin. Thromb. Hemostasis, 1996, vol. 22, pp. 309–315.
Van Nieuw Amerongen, G.P., Van Delft S., Vermeer, M.A., Collard, J.G., and van Hinsbergh, V.W., Activation of RhoA by Thrombin in Endothelial Hyperpermeability: Role of Rho Kinase and Protein Tyrosine Kinases, Circ. Res., 2000, vol. 87, pp. 335–340.
Groeneveld, A.B., Vascular Pharmacology of Acute Lung Injury and Acute Respiratory Distress Syndrome, Vascul. Pharmacol., 2002, vol. 39, pp. 247–256.
Smurova, K.M., Birukova, A.A., Garcia, J., Vorobyev, I.A., Alieva, I.B., and Verin, A.D., Thrombin-Induced Rearrangement of the Microtubule System in Lung Endothelium Cells, Tsitologiya (Rus.), 2004, vol. 46, no. 8, pp. 695–703.
Smurova, K.M., Birukova, A.A., Verin, A.D., and Alieva, I.B., Microtubule System in Endothelial Barrier Dysfunction: Disassembly of Peripheral Microtubules and Microtubule Reorganization in Internal Cytoplasm, Cell and Tissue Biology, 2008, vol. 2, no. 1, pp. 45–52.
Cattan, C.E. and Oberg, K.C., Vinorelbine Tartrate-Induced Pulmonary Edema Confirmed on Rechallenge, Pharmacotherapy, 1999, vol. 19, no. 8, pp. 992–994.
Uoshima, N., Yoshioka, K., Tegoshi, H., Wada, S., Fujiwara, Y., Satake, N., Kasamatsu, Y., and Yokoho, S., Acute Respiratory Failure Caused by Vinorelbine Tartrate in a Patient with Non-Small Cell Lung Cancer, Intern. Med., 2001, vol. 40, no. 8, pp. 779–782.
George, Ph., Journey, L.J., and Goldstein, M., Effect of Vincristine on the Fine Structure of HeLa Cell during Mitosis, J. Natl. Cancer Inst., 1965, vol. 35, pp. 355–365.
Krishan, A., Fine Structure of the Kinetochore in Vinblastine Sulphate-Treated Cells, J. Ultrastruct. Res., 1968, vol. 23, pp. 124–143.
Thilagaratnam, C.N. and Main, J.H., Mitostatic Action of Vinblastine Sulphate on Oral Epithelia of Hamsters, J. Oral Pathol., 1972, vol. 1, no. 2, pp. 84–88.
Zuckerberg, C. and Solari, A.J., Centriolar Changes Induced by Vinblastine Sulphate in the Seminiferous Epithelium of the Mouse, Exp. Cell. Res., 1973, vol. 76, pp. 470–475.
Alieva, I.B., Gorgidze, L.A., Komarova, Yu.A., Chernobelskaya, O.A., and Vorobjev, I.A., Experimental Model for Studying the Primary Cilia in Tissue Cultured Cells, Membr. Cell. Biol., 1999, vol. 12, no. 6, pp. 895–905.
Verin, A.D., Birukova, A., Wang, P., Liu, F., Becker, P., Birukov, K., and Garcia, J.G., Microtubule Disassembly Increases Endothelial Cell Barrier Dysfunction: Role of MLC Phosphorylation, Am. J. Physiol.: Lung Cell Mol. Physiol., 2001, vol. 281, pp. L565–L574.
Hirsch, K., Danilenko, M., Giat, J., Miron, T., Rabinkov, A., Wilchek, M., Mirelman, D., Levy, J., and Sharoni, Y., Effect of Purified Allicin, the Major Ingredient of Freshly Crushed Garlic, on Cancer Cell Proliferation, Nutr. Cancer, 2000, vol. 38, no. 2, pp. 245–254.
Oommen, S., Anto, R.J., Srinivas, G., and Karunagaran, D., Allicin (from Garlic) Induces Caspase-Mediated Apoptosis in Cancer Cells, Eur. J. Pharmacol., 2004, vol. 485, nos. 1–3, pp. 97–103.
Prager-Khoutorsky, M., Goncharov, I., Rabinkov, A., Mirelman, D., Geiger, B., and Bershadsky, A.D., Allicin Inhibits Cell Polarization, Migration and Division via Its Direct Effect on Microtubules, Cell. Motil. Cytoskeleton, 2007, vol. 64, no. 5, pp. 321–337.
Aznar, S., Fernandez-Valeron, P., Espina, C., and Lacal, J.C., Rho GTPases: Potential Candidates for Anticancer Therapy, Cancer Lett., 2004, vol. 206, no. 2, pp. 181–191.
Zhang, B., Rho GDP Dissociation Inhibitors As Potential Targets for Anticancer Treatment, Drug Resist. Updat. 2006, vol. 9, no. 3, pp. 134–141.
Fritz, G. and Kaina, B., Rho GTPases: Promising Cellular Targets for Novel Anticancer Drugs, Curr. Cancer Drug Targets, 2006, vol. 6, no. 1, pp. 1–14.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © K.M. Smurova, A.A. Birukova, A.D. Verin, I.B. Alieva, 2008, published in Biologicheskie Membrany, 2008, Vol. 25, No. 3, pp. 181–190.
Rights and permissions
About this article
Cite this article
Smurova, K.M., Birukova, A.A., Verin, A.D. et al. Dose-dependent effect of nocodazole on endothelial cell cytoskeleton. Biochem. Moscow Suppl. Ser. A 2, 119–127 (2008). https://doi.org/10.1134/S1990747808020049
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747808020049