Abstract
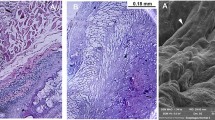
The use of recellularized matrices allows obtaining tissue-engineered structures that largely reproduce the morphofunctional features of native organs and tissues. Doubts as to the advisability of preimplantation recellularization of the scaffold, which are associated with the death of prepopulation cells on the scaffold in vivo, require studying the behavior of cells on plastic and biological implants after recellularization. Rat esophagus that had undergone detergent-enzymatic decellularization was repopulated with GFP-positive cells intensely fluorescing in the green spectrum, which allowed us to trace the status of the initial cell population during cultivation on plastic and after transplantation, determine the viability and metabolic activity of cells, and conduct fluorescent detection of the cells on the scaffold before and after implantation. There were no data on the apparent cell proliferation on the matrix; however, there were indirect indications of metabolic activity and GFP synthesis by the cells that populated the scaffold before implantation.
Similar content being viewed by others
Abbreviations
- MSC:
-
mesenchymal stromal cell
- GFP:
-
green fluorescent protein (a protein consisting of 238 amino acids with bright fluorescence in the green spectrum).
References
Bagaeva, V.V., Popova, V.M., Pashkova, G.S., Isadzhanyan, K.E., Nikitin, V.V., and Zhilenkov, E.L., The study the efficacy and safety of antimicrobial agents, Issled. Prakt. Med., 2015, vol. 2, no. 3, pp. 35–42.
Basu, J., Mihalko, K.L., Payne, R., Rivera, T., Knight, T., Genheimer, C.W., Guthrie, K.I., Sangha, N., Jayo, M.J., Jain, D., Bertram, T.A., and Ludlow, J.W., Extension of bladder-based organ regeneration platform for tissue engineering of esophagus, Med. Hypotheses, 2012, vol. 78, pp. 231–234.
Basu, J., Mihalko, K.L., Rivera, E.A., Guthrie, K.I., Genheimer, C.W., Sangha, N., and Ludlow, J.W., Tissue engineering of esophagus and small intestine in rodent injury models, Methods Mol. Biol., 2013, vol. 1001, pp. 311–324.
Becquart, P., Cambon-Binder, A., Monfoulet, L.E., Bourguignon, M., Vandamme, K., Bensidhoum, M., Petite, H., and Logeart-Avramoglou, D., Ischemia is the prime but not the only cause of human multipotent stromal cell death in tissue-engineered constructs in vivo, Tissue Eng. Part A, 2012, vol. 18, pp. 2084–2094.
Christ, T., Dohmen, P.M., Holinski, S., Schönau, M., Heinze, G., and Konertz, W., Suitability of the rat subdermal model for tissue engineering of heart valves, Med. Sci. Monit. Basic. Res., 2014, vol. 20, pp. 194–199.
Deng, M., Chang, Z., Hou, T., Dong, S., Pang, H., Li, Z., Luo, F., Xing, J., Yu, B., Yi, S., and Xu, J., Sustained release of bioactive protein from a lyophilized tissue-engi-neered construct promotes the osteogenic potential of mesenchymal stem cells, J. Orthop. Res., 2016, vol. 34, pp. 386–394.
Gubareva, E.A., Sjöqvist, S., Gilevich, I.V., Sotnichenko, A.S., Kuevda, E.V., Lim, M.L., Feliu, N., Lemon, G., Danilenko, K.A., Nakokhov, R.Z., Gumenyuk, I.S., Grigoriev, T.E., Krasheninnikov, S.V., Pokhotko, A.G., Basov, A.A., Dzhimak, S.S., Gustafsson, Y., Bautista, G., Rodríguez, A.B., Pokrovsky, V.M., Jungebluth, P., Chvalun, S.N., Holterman, M.J., Taylor, D.A., and Macchiarini, P., Orthotopic transplantation of a tissue engineered diaphragm in rats, Biomaterials, 2016, vol. 77, pp. 320–335.
Kuevda, E.V., Gubareva, E.A., Sotnichenko, A.S., Gumenyuk, I.S., Gilevich, I.V., Polyakov, I.S., Porkhanov, V.A., Alekseenko, S.N., and Macchiarini, P., Experience of perfusion recellularization of biological lung scaffold in rats, Vestn. Transplantol. Iskusstv. Org., 2016, vol. 18, no. 1, pp. 38–44.
Kuevda, E.V., Gubareva, E.A., Sotnichenko, A.S., Gumenyuk, I.S., Gilevich, I.V., and Nakokhov, R.Z., Comparative characterization of assessive methods of the cytotoxic properties of biological scaffolds, Geny Kletki, 2017, vol. 12, no. 1, pp. 57–61.
Lim, M.L., Ooi, B.N., Jungebluth, P., Sjöqvist, S., Hultman, I., Lemon, G., Gustafsson, Y., Asmundsson, J., Baiguera, S., Douagi, I., Gilevich, I., Popova, A., Haag, J.C., Rodríguez, A.B., Lim, J., Liedén, A., Nordenskjöld, M., Alici, E., Baker, D., Unger, C., Luedde, T., Vassiliev, I., Inzunza, J., Ahrlund-Richter, L., and Macchiarini, P., Characterization of stem-like cells in mucoepidermoid tracheal paediatric tumor, PLoS One, 2014, vol. 9, pp. 1–12.
Londono, R. and Badylak, S.F., Regenerative medicine strategies for esophageal repair, Tissue Engineering Part B, 2015, vol. 21, pp. 393–410.
Lopes, M.F., Cabrita, A., Ilharco, J., Pessa, P., and Patrıcio, J., Grafts of porcine intestinal submucosa for repair of cervical and abdominal esophageal defects in the rat, J. Invest. Surg., 2006, vol. 19, pp. 105–111.
Ludman, L. and Spitz, L., Quality of life after gastric transposition for oesophageal atresia, J. Pediatr. Surg., 2003, vol. 38, pp. 53–57.
Monastyrskaya, E.A., Lyamina, S.V., and Malyshev, I.Yu., Ml and M2 phenotypes of activated macrophages and their role in immune response and pathology, Patogenez, 2008, vol. 6, no. 4, pp. 31–39.
Tan, B., Wei, R.Q., Tan, M.Y., Luo, J.C., Deng, L., Chen, X.H., Hou, J.L., Li, X.Q., Yang, Z.M., and Xie, H.Q., Tissue engineered esophagus by mesenchymal stem cell seeding for esophageal repair in a canine model, J. Surg. Res., 2013, vol. 182, pp. 40–48.
Tao, R., Sun, T.J., Han, Y.Q., Xu, G., Liu, J., and Han, Y.F., Optimization of in vitro cell labeling methods for human umbilical cord-derived mesenchymal stem cells, Eur. Rev. Med. Pharmacol. Sci., 2014, vol. 18, pp. 1127–1134.
Totonelli, G., Maghsoudlou, P., Garriboli, M., Riegler, J., Orlando, G., Burns, A.J., Sebire, N.J., Smith, V.V., Fishman, J.M., Ghionzoli, M., Turmaine, M., Birchall, M.A., Atala, A., Soker, S., Lythgoe, M.F., Seifalian, A., Pierro, A., Eaton, S., and De Coppi, P., A rat decellularized small bowel 15 scaffold that preserves villus-crypt architecture for intestinal regeneration, Biomaterials, 2012, vol. 33, pp. 3401–3410.
Totonelli, G., Maghsoudlou, P., Georgiades, F., Garribol, M., Koshy, K., Turmaine, M., Ashworth, M., Sebire, N.J., Pierro, A., Eaton, S., and De Coppi, P., Detergent enzymatic treatment for the development of a natural acellular matrix for oesophageal regeneration, Pediatr. Surg. Int., 2013, vol. 29, pp. 87–95.
van Vollenstee, F.A., Jackson, C., Hoffmann, D., Potgieter, M., Durandt, C., and Pepper, M.S., Human adipose derived mesenchymal stromal cells transduced with gfp lentiviral vectors: assessment of immunophenotype and differentiation capacity in vitro, Cytotechnology, 2016, vol. 68, pp. 2049–2060.
Yamamoto, Y., Nakamura, T., Shimizu, Y., Matsumoto, K., Takimoto, Y., Kiyotani, T., Sekine, T., Ueda, H., Liu, Y., and Tamura, N., Intrathoracic esophageal replacement in the dog with the use of an artificial esophagus composed of a collagen sponge with a doublelayered silicone tube, J. Thorac. Cardiovasc. Surg., 1999, vol. 118, pp. 276–286.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.V. Kuevda, E.A. Gubareva, R.Z. Nakokhov, I.S. Gumenyuk, A.S. Sotnichenko, D.P. Puzanov, 2017, published in Tsitologiya, 2017, Vol. 59, No. 10, pp. 711–717.
Rights and permissions
About this article
Cite this article
Kuevda, E.V., Gubareva, E.A., Nakokhov, R.Z. et al. The Effectiveness of Preimplantation Recellularization of Acellular Rat-Esophagus Matrices Using GFP-Positive Cells. Cell Tiss. Biol. 12, 160–166 (2018). https://doi.org/10.1134/S1990519X18020037
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X18020037