Abstract
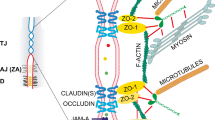
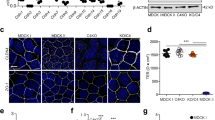
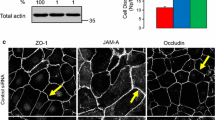
The dynamics of the actin cytoskeleton spatial organization and transepithelial electric resistance (TEER) in the MDCK1 cell monolayer exposed to arginine–vasopressin (AVP) and forskolin, a protein kinase A (PKA) activator, have been studied. These physiologically active substances are shown to depolymerize filamentous actin in MDCK1 cells (in both the apical and basal cytoplasm) and, concurrently, to considerably decrease the TEER of the cell monolayer. A decrease in TEER suggests an increase in the ion current through the cell monolayer. Correspondingly, the created ion gradient stimulates AVP-sensitive water flow. To clarify the routes of ions and water in MDCK monolayer, the localization of claudin-1 and -2 in tight junctions of ATCC (American Type Culture Collection) MDCK (a low TEER) and MDCK1 (a high TEER) cells was studied by immunofluorescence assay. Claudin-1 and -2 are detectable in the tight junctions of ATCC MDCK cells; however, the tight junctions of MDCK1 cells contain only claudin-1, whereas poreforming claudin-2 is absent. The exposure of MDCK1 cells to forskolin fails to change the distribution of the studied claudins, thereby suggesting that a decrease in TEER caused by forskolin is associated with a change in transcellular, rather than paracellular, permeability of the monolayer
Similar content being viewed by others
Abbreviations
- cAMP:
-
cyclic adenosine monophosphate
- ATCC:
-
American Type Culture Collection
- AVP:
-
arginine vasopressin
- MR:
-
mineralocorticoid receptor
- PKA:
-
protein kinase A
- ENaC:
-
epithelial Na channel
- TEER:
-
transepithelial electric resistance
References
Ammer, A.G. and Weed, S.A., Cortactin branches out: roles in regulating protrusive actin dynamics, Cell Motil. Cytoskel., 2008, vol. 65, pp. 687–707.
Anderson, J., Van Itallie, C., and Fanning, A., Setting up a selective barrier at the apical junction complex, Curr. Opin. Cell Biol., 2004, vol. 1–16, pp. 140–145.
Angelow, S. and Yu, A.S., Claudins and paracellular transport: an update, Curr. Opin. Nephrol. Hypertens., 2007, vol. 16, pp. 459–464.
Ashkroft, F.M., Ion Channels and Disease, San Diego, CA: Academic Press, 2000, pp. 1–502.
Bankir, L., Fernandes, S., Bardoux, P., Bouby, N., and Bichet, D.G., Vasopressin-V2 receptor stimulation reduces sodium excretion in healthy humans, J. Amer. Soc. Nephrol., 2005, vol. 16, pp. 1920–1928.
Barberis, C., Mouillac, B., and Durroux, T., Structural bases of vasopressin/oxytocin receptor function, J. Endocrinol., 1998, vol. 156, pp. 223–229.
Barker, G. and Simmons, N., Identification of two strains of cultured canine renal epithelial cells (MDCK cells) which display entirely different physiological properties, Quart. J. Exp. Physiol., 1981, vol. 66, pp. 61–72.
Bens, M., Chassin, C., and Vandewalle, A., Regulation of NaCl transport in the renal collecting duct: lessons from cultured cells, Eur. J. Physiol., 2006, vol. 453, pp. 133–146.
Benson, K., Cramer, S., and Galla, H.J., Impedance-based cell monitoring: barrier properties and beyond, Fluids Barriers CNS, 2013, vol. 10, p. 5.
Berdiev, B.K., Prat, A.G., Cantiello, H.F., Dennis, A., Ausiello, D.A., Fuller, C.M., Jovov, B., Benos, D.J., and Ismailov, I.I., Regulation of epithelial sodium channels by short actin filaments, J. Biol. Chem., 1996, vol. 271, pp. 17704–17710.
Berdiev, B.K., Latorre, R., Benos, D.J., and Ismailov, I.I., Actin modifies Ca2+ block of epithelial Na+ channels in planar lipid bilayers, Biophys. J., 2001, vol. 80, pp. 2176–2186.
Blazer-Yost, B., Record, R., and Oberleithner, H., Characterization of hormone-stimulated Na+ transport in a highresistance clone of the MDCK cell line, Eur. J. Physiol., 1996, vol. 432, pp. 685–691.
Brown, D., Breton, S., Ausiello, D.A., and Marshansky, V., Sensing, signaling and sorting events in kidney epithelial cell physiology, Traffic, 2009, vol. 10, pp. 275–284.
Bugaj, V., Pochynyuk, O., and Stockand, J.D., Activation of the epithelial Na channel in the collecting duct by vasopressin contributes to water reabsorption, Amer. J. Physiol., 2009, vol. 297, pp. F1411–F1418.
Butterworth, M.B., Edinger, R.S., Johnson, J.P, and Frizzell, R.A., Acute ENaC stimulation by cAMP in a kidney cell line is mediated by exocytic insertion from a recycling channel pool, J. Gen. Physiol., 2005, vol. 125, pp. 81–101.
Bystriansky, J. and Kaplan, J., Sodium pump localization in epithelia, J Bioenerg. Biomembr., 2007, vol. 39, pp. 373–378.
Cantiello, H.F., Stow, J.L., Prat, A.G., and Ausiello, D.A., Actin filaments regulate epithelial Na+ channel activity, Amer. J. Physiol., 1991, vol. 261, pp. C882–C888.
Caplan, M.J., Anderson, H.C., Palade, G.E., and Jamieson, J.D., Intracellular sorting and polarized cell surface delivery of (Na+,K+)ATPase, an endogenous component of MDCK cell basolateral plasma membranes, Cell, 1986, vol. 46, pp. 623–631.
Cereijido, M., Robbins, E.S., Dolan, W.J., Rotunno, C.A., and Sabatini, D.D., Polarized monolayers formed by epithelial cells on a permeable and translucent support, J. Cell Biol., 1978, vol. 77, pp. 853–880.
Cereijido, M., Ehrenfeld, J., Fernindez-Castelo, S., and Meza, I., Fluxes, junctions, and blisters in cultured monolayers of epithelioid cells (MDCK), Ann. N.Y. Acad. Sci., 1981a, vol. 372, pp. 422–441.
Cereijido, M., Meza, I., and Martinez-Palomo, A., Occluding junctions in cultured epithelial monolayers, Amer. J. Physiol., 1981b, vol. 240, pp. C96–C102.
Chen, S.Y., Bhargava, A., Mastroberardino, L., Meijer, O.C., Wang, J., Buse, P., Firestone, G., Verrey, F., and Pearce, D., Epithelial sodium channel regulated by aldosteroneinduced protein sgk, Proc. Natl. Acad. Sci. USA, 1999, vol. 96, pp. 2514–2519.
Copeland, S.J., Berdiev, B.K., Ji, H.L., Lockhart, J., Parker, S., Fuller, C.M., and Benos, D.J., Regions in the carboxy terminus of alpha-bENaC involved in gating and functional effects of actin, Amer. J. Physiol., 2001, vol. 281, pp. C231–C240.
Eaton, D.C., Malik, B., Bao, H..F, Yu, L., and Jain, L., Regulation of epithelial sodium channel trafficking by ubiquitination, Proc. Amer. Thorac. Soc., 2010, vol. 7, pp. 54–64.
Elkouby-Naor, L. and Ben-Yosef, T., Functions of claudin tight junction proteins and their complex interactions in various physiological systems, Int. Rev. Cell Mol. Biol., 2010, vol. 279, pp. 1–32.
Erlij, D., De, Smet, P., Mesotten, D., and Van Driessche, W., Forskolin increases apical sodium conductance in cultured toad kidney cells (A6) by stimulating membrane insertion, Pflügers Arch., 1999, vol. 438, pp. 195–204.
Frindt, G. and Burg, M.B., Effect of vasopressin on sodium transport in renal cortical collecting tubules, Kidney Int., 1972, vol. 1, pp. 224–231.
Furuse, M., Furuse, K., Sasaki, H., and Tsukita, S., Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin–Darby canine kidney I cells, J. Cell Biol., 2001, vol. 153, pp. 263–272.
Garty, H. and Edelman, I.S., Amiloride-sensitive trypsinization of apical sodium channels, analysis of hormonal regulation of sodium transport in toad bladder, J. Gen. Physiol., 1983, vol. 81, pp. 785–803.
Garty, H. and Palmer, L.G., Epithelial sodium channels: function, structure, and regulation, Physiol. Rev., 1997, vol. 77, pp. 359–396.
Gekle, M, Wunsch, S, Oberleithner, H, and Silbernagl, S., Characterization of two MDCK-cell subtypes as a model system to study principal cell and intercalated cell properties, Pflugers Arch., 1994, vol. 428, pp. 157–162.
Gorshkov, A.N. and Komissarchik, Ia.Iu., Structural rearrangements of microfilaments and granular cells of the frog bladder during induction of water transport: fluorescent microscopic, immunocytochemical and electron microscopic studies, Tsitologiia, 1997, vol. 39, 11, pp. 1032–1037.
Hall, A. and Nobes, C.D., Rho GTPases: molecular switches that control the organization and dynamics of the actin cytoskeleton, Philos. Trans. R. Soc. Lond. B Biol. Sci., 2000, vol. 355, pp. 965–970.
Handler, J., Use of cultured epithelia to study transport and its regulation, J. Exp. Biol., 1983, vol. 106, pp. 55–69.
Hays, R., Condeelis, J., Gao, Y., Simon, H., Ding, G., and Franki, N., The effect of the vasopressin on the cytoskeleton of the epithelial cell, Pediatr. Nephrol., 1993, vol. 7, pp. 672–679.
Ilatovskaya, D.V., Pavlov, T.S., Levchenko, V., Negulyaev, Y.A., and Staruschenko, A., Cortical actin binding protein cortactin mediates ENaC activity via Arp2/3 complex, FASEB J., 2011, vol. 25, pp. 2688–2699.
Inai, T., Kobayashi, J., and Shibata, Y., Claudin-1 contributes to the epithelial barrier function in MDCK cells, Eur. J. Cell Biol., 1999, vol. 78, pp. 849–855.
Ivanova, L.N., Vasopressin: cellular and molecular aspects of its antidiuretic effect, Vestn. Ross. Akad. Med. Nauk, 1999, vol. 3, pp. 40–45.
Jeffries, W.B., Wang, Y., and Pettinger, W.A., Enhanced vasopressin (V2-receptor)-induced sodium retention in mineralocorticoid hypertension, Amer. J. Physiol., 1988, vol. 254, pp. F739–F746.
Jovov, B., Tousson, A., Ji, H.L., Keeton, D., Shlyonsky, V., Ripoll, P.J., Fuller, C.M., and Benos, D.J., Regulation of epithelial Na(+) channels by actin in planar lipid bilayers and in the Xenopus oocyte expression system, J. Biol. Chem., 1999, vol. 274, pp. 37845–37854.
Karpushev, A.V., Ilatovskaya, D.V., Pavlov, T.S., Negulyaev, Y.A., and Staruschenko, A., Intact cytoskeleton is required for small G protein dependent activation of the epithelial Na+ channel, PLoS ONE, 2010, vol. 5, p. e8827.
Karpushev, A.V., Levchenko, V., Ilatovskaya, D.V., Pavlov, T.S., and Staruschenko, A., Novel role of Rac1/WAVE signaling mechanism in regulation of the epithelial Na+ channel, Hypertension, 2011, vol. 2011 57, pp. 996–1002.
Klussmann, E., Tamma, G., Lorenz, D., Wiesner, B., Maric, K., Hofmann, F., Aktories, K., Valenti, G., and Rosenthal, W., An inhibitory role of Rho in the vasopressinmediated translocation of aquaporin-2 into cell membranes of renal principal cells, J. Biol. Chem., 2001, vol. 276, pp. 20451–20457.
Komissarchik, Ia.Iu. and Snigirevskaia, E.S., The participation of intracellular membranes in forming highly permeable domains in the plasma membrane of epithelial cells during the vasopressin stimulation of water transport, Tsitologiia, 1991, vol. 33, 11, pp. 135–140.
Krause, G., Winkler, L., Mueller, S.L., Haseloff, R.F., Piontek, J., and Blasig, I.E., Structure and function of claudins, Biochim. Biophys. Acta, 2008, vol. 1778, pp. 631–645.
Kreisberg, J., and Wilson, P., Renal cell culture, J. Electron Microsc. Tech., 1988, vol. 9, pp. 235–263.
Loffing, J. and Korbmacher, C., Regulated sodium transport in the renal connecting tubule (CNT) via the epithelial sodium channel (ENaC), Pflugers Arch., 2009, vol. 458, pp. 111–135.
Loffing, J., Zecevic, M., Féraille, E., Kaissling, B., Asher, C., Rossier, B.C., Firestone, G.L., Pearce, D., and Verrey, F., Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system: possible role of SGK, Amer. J. Physiol., 2001, vol. 280, pp. F675–F682.
Marunaka, Y., Hormonal and osmotic regulation of NaCl transport in renal distal nephron epithelium, Japan. J. Physiol., 1997, vol. 47, pp. 499–511.
Marunaka, Y. and Eaton, D.C., Effects of vasopressin and cAMP on single amiloride-blockable Na channels, Amer. J. Physiol., 1991, vol. 260, pp. C1071–C1084.
Mazzochi, C., Benos, D.J., and Smith, P.R., Interaction of epithelial ion channels with the actin-based cytoskeleton, Amer. J. Physiol., 2006a, vol. 291, pp. F1113–F1122.
Mazzochi, C., Bubien, J.K., Smith, P.R., and Benos, D.J., The carboxyl terminus of the alpha-subunit of the amiloride-sensitive epithelial sodium channel binds to F-actin, J. Biol. Chem., 2006b, vol. 281, pp. 6528–6538.
McCarthy, K.M., Francis, S.A., McCormack, J.M., Lai, J., Rogers, R.A., Skare, I.B., Lynch, R.D., and Schneeberger, E.E., Inducible expression of claudin-1-myc but not occludin-VSV-G results in aberrant tight junction strand formation in MDCK cells, J. Cell Sci., 2000, vol. 113, pp. 3387–3398.
Mel’nitskaia, A.V., Krutetskaia, Z.I., and Lebedev, O.E., Structural and functional organization of Na+ transport in epithelial systems. I. Epithelial Na+ channels, Tsitologiia, 2006, vol. 48, 10, pp. 817–840.
Mishler, D., Kraut, J., and Nagami, G., AVP reduces transepithelial resistance across IMCD cell monolayers, Amer. J. Physiol., 1990, vol. 258, pp. F1561–F1568.
Morris, R.G. and Schafer, J.A., cAMP increases density of ENaC subunits in the apical membrane of MDCK cells in direct proportion to amiloridesensitive Na(+) transport, J. Gen. Physiol., 2002, vol. 120, pp. 71–85.
Muller, J. and Kachadorian, W., Aggregate-carrying membranes during ADH-stimulation and washout in toad bladder, Amer. J. Physiol., 1984, vol. 247, pp. C90–C98.
Nakamura, F., Stossel, T.P., and Hartwig, J.H., The filamins: organizers of cell structure and function, Cell Adh. Migr., 2011, vol. 5, pp. 160–169.
Nejsum, L., Zelenina, M., Aperia, A., Frokiaer, J., and Nielsen, S., Bidirectional regulation of AQP2 trafficking and recycling: involvement of AQP2-S256 phosphorylation, Amer. J. Physiol., 2005, vol. 288, pp. F930–938.
Nielsen, S., Chou, C., Marples, D., Christensen, E., Kishore, B., and Knepper, M., Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane, Proc. Natl. Acad. Sci. USA, 1995, vol. 92, pp. 1013–1017.
Niisato, N. and Marunaka, Y., Activation of the Na+-K+ pump by hyposmolality through tyrosine kinase-dependent Cl– conductance in Xenopus renal epithelial A6 cells, J. Physiol., 1999, vol. 518, pp. 417–432.
Oberleithner, H., Aldosterone-regulated ion transporters in the kidney, Klin. Wochenschr., 1990, vol. 68, pp. 1087–1090.
Pinaev, G.P., Contractile systems of a cell: from muscle contraction to regulation of cell functions, Tsitologiia, 2009, vol. 51, 3, pp. 172–181.
Pochynyuk, O., Medina, J., Gamper, N., Genth, H., Stockand, J.D., and Staruschenko, A., Rapid translocation and insertion of the epithelial Na+ channel in response to RhoA signaling, J. Biol. Chem., 2006, vol. 281, pp. 26520–26527.
Prat, A.G., Bertorello, A.M., Ausiello, D.A., and Cantiello, H.F., Activation of epithelial Na+ channels by protein kinase A requires actin filaments, Amer. J. Physiol., 1993, vol. 265, pp. C224–C233.
Rehn, M., Weber, W.-M., and Clauss, W., Role of the cytoskeleton in stimulation of Na+ channels in A6 cells by changes in osmolality, Eur. J. Physiol., 1998, vol. 436, pp. 270–279.
Reif, M.C., Troutman, S.L., and Schafer, J.A., Sodium transport by rat cortical collecting tubule, effects of vasopressin and desoxycorticosterone, J. Clin. Invest., 1986, vol. 77, pp. 1291–1298.
Reifenberger, M.S., Yu, L., Bao, H.-F., Duke, B.J., Liu, B.-C., Ma, H.-P., Alli, A.A., Eaton, D.C., and Alli, A., Cytochalasin E alters the cytoskeleton and decreases ENaC activity in Xenopus 2F3 cells, Amer. J. Physiol., 2014, vol. 307, pp. F86–F95.
Richardson, J., Scalera, V., and Simmons, N., Identification of two strains of MDCK cells which resemble separate nephron tubule segments, Biochim. Biophys. Acta, 1981, vol. 673, pp. 26–36.
Saier, M.H., Jr., Boerner, P., Grenier, F.C., McRoberts, J.A., Rindler, M.J., and Taub, M., Sodium entry pathways in renal epithelial cell lines, Miner. Electrolyte Metab., 1986, vol. 12, pp. 42–50.
Saxena, S.K. and Kaur, S., Regulation of epithelial ion channels by Rab GTPases, Biochem. Biophys. Res. Commun., 2006, vol. 351, pp. 582–587.
Schafer, J.A. and Troutman, S.L., cAMP mediates the increase in apical membrane Na+ conductance produced in rat CCD by vasopressin, Amer. J. Physiol., 1990, vol. 259, pp. F823–F831.
Simon, H., Gao, Y., Franki, N., and Hays, R., Vasopressin depolymerizes apical F-actin in rat inner medullary collecting duct, Amer. J. Physiol., 1993, vol. 265, pp. C757–C762.
Skorecki, K., Brown, D., Ercolani, L., and Ausiello, D., Molecular mechanisms of vasopressin action in the kidney, in Handbook of Physiology, Sect. 8: Renal Physiology, Windhager, E., Ed., New York, Oxford, 1992, pp. 1185–1218.
Srinivasan, B., Kolli, A.R., Esch, M.B., Abaci, H.E., Shuler, M.L., and Hickman, J.J., TEER measurement mechniques for in vitro barrier model systems, J. Lab. Autom., 2015, vol. 20, pp. 107–126.
Staruschenko, A., Patel, P., Tong, Q., Medina, J.L., and Stockand, J.D., Ras activates the epithelial Na(+) channel through phosphoinositide 3-OH kinase signaling, J. Biol. Chem., 2004, vol. 279, pp. 37771–37778.
Staruschenko, A., Pochynyuk, O.M., Tong, Q., and Stockand, J.D., Ras couples phosphoinositide 3-OH kinase to the epithelial Na+ channel, Biochim. Biophys. Acta, 2005, vol. 1669, pp. 108–115.
Staub, O., Abriel, H., Plant, P., Ishikawa, T., Kanelis, V., Saleki, R., Horisberger, J.D., Schild, L., and Rotin, D., Regulation of the epithelial Na+ channel by Nedd4 and ubiquitination, Kidney Int., 2000, vol. 57, pp. 809–815.
Stockand, J.D., Vasopressin regulation of renal sodium excretion, Kidney Int., 2010, vol. 78, pp. 849–856.
Tanner, C., Frambach, D.A., and Misfeldt, D.S., Biophysics of domes formed by the renal cell line Madin–Darby canine kidney, Fed. Proc., 1984, vol. 43, pp. 2217–2220.
Van Itallie, C., Holmes, J., Bridges, A., Gookin, J., Coccaro, M., Proctor, W., Colegio, O., and Anderson, J., The density of small tight junction pores varies among cell types and increased expression of claudin-2, J. Cell Sci., 2008, vol. 121, pp. 298–305.
Verhovez, A., Williams, T.A., Monticone, S., Crudo, V., Burrello, J., Galmozzi, M., Covella, M., Veglio, F., and Mulatero, P., Genomic and non-genomic effects of aldosterone, Curr. Sign. Transd. Ther., 2012, vol. 7, pp. 132–141.
Verrey, F., Kraehenbuhl, J.P., and Rossier, B.C., Aldosterone induces a rapid increase in the rate of Na,K-ATPase gene transcription in cultured kidney cells, Mol. Endocrinol., 1989, vol. 3, pp. 1369–1376.
Vinciguerra, M., Mordasini, D., Vandewalle, A., and Feraille, E., Hormonal and nonhormonal mechanisms of regulation of the Na,K-pump in collecting duct principal cells, Semin. Nephrol., 2005, vol. 25, pp. 312–321.
Wang, Q., Dai, X.Q., Li, Q., Tuli, J., Liang, G., Li, S.S., and Chen, X.Z., Filamin interacts with epithelial sodium channel and inhibits its channel function, J. Biol. Chem., 2013, vol. 288, pp. 264–273.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.N. Gorshkov, M.R. Zaitseva, E.S. Snigirevskaya, Ya.Yu. Komissarchik, 2015, published in Tsitologiya, 2015, Vol. 57, No. 11, pp. 796–807.
Rights and permissions
About this article
Cite this article
Gorshkov, A.N., Zaitseva, M.R., Snigirevskaya, E.S. et al. Functional linkage between the transport characteristics of the MDCK1 cell monolayer and their actin cytoskeleton organization. Cell Tiss. Biol. 10, 133–144 (2016). https://doi.org/10.1134/S1990519X1602005X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X1602005X