Abstract
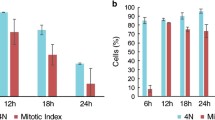
Analyzed in this study is the organization of mitotic spindle poles in CHO-K1 cells dividing after treatment with etoposide (1 h, 25 μM). At various periods after the treatment, we studied the following: (1) the distribution of γ-tubulin in mitotic cells by immunofluorescent staining, (2) the level of post-translational modification of α-tubulin in spindle microtubules by immunoelectron microscopy, and (3) the ultrastructure of mitotic apparatus poles by standard electron microscopy. 48 h after the addition of etoposide, disturbances in the ultrastructure of mitotic spindle poles were observed in etoposide-treated CHO-K1 cells with both bipolar and with multipolar mitotic apparatuses. The increased number of centrioles was unevenly distributed between the mitotic spindle poles; some centrioles did not take an obvious part in the mitotic spindle organization and differed in their number of outgrowing microtubules. Most centrioles were without fibrillar halos. Immunoelectron microscopy showed the differences in the staining of the poles of a multipolar spindle within one cell with antibodies to tyrosinated α-tubulin, whereas the staining of cells with antibodies to acetylated α-tubulin did not reveal such differences. Immunofluorescence staining for γ-tubulin also indicated differing organizations of poles in the same spindle. Our data findings provided the first evidence that the pattern of immunostaining and ultrastructure of mitotic apparatus poles can differ in cells dividing at various time periods after the action of etoposide.
Similar content being viewed by others
References
Balashova, E.E., Ryaskina, S.S., Vinogradova, T.M., and Bystrevskaya, V.B., Reorganization of Mitotic Apparatus in the Etoposide-treated CHO-K1 cells Precedes Apoptotic Death, Tsitologiya, 2008, vol. 2, no. 3, pp. 282–289.
Abal, M., Souto, A.A., Amat-Guerri, F., Acuna, A.U., Andreu, J.M., and Barasoain, I., Centrosome and Spindle Pole Microtubules Are Main Targets of a Fluroescent Taxoid Inducing Cell Death. Cell. Motil. Cytoskelet., 2001, vol. 49, pp. 1–15.
Balczon, R., Bao, L., Zimmer, W.E., Brown, K., Zinkowski, R.P., and Brinkley, B.R., Dissociation of Centrosome Replication Events from Cycles of DNA Synthesis and Mitotic Division in Hydroxyurea-Arrested CHO Cells, J. Cell Biol., 1995, vol. 130, pp. 105–115.
Bharadwaj, R. and Yu, H., The Spindle Checkpoint, Aneuploidy, and Cancer, Oncogene, 2004, vol. 23, 2016–2027.
Bre, M-H., Kreis, T.E., and Karsenti, E., Control of Microtubules Nucleation and Stability in MDCK Cells: the Occurrence of Noncentrosomal, Stable Detyrosinated Microtubules, J. Cell Biol., 1987, vol. 105, pp. 1283–1296.
Brinkley, B.R., Managing the Centrosome Numbers Game: From Chaos to Stability in Cancer Cell Division, Trends Cell Biol. 2001, vol. 11, pp. 18–21.
Brinkley, B.R. and Goepfert, T.M., Supernumerary Centrosomes and Cancer: Boveri’s Hypothesis Resurrected, Cell. Motil. Cytoskelet., 1998, vol. 41, pp. 281–288.
Bystrevskaya, V.B., Lobova, T.V., Smirnov, V.N., Makarova, N.E., and Kushch, A.A., Centrosome Injury in Cells Infected with HCMV, J. Struct. Biol., 1997, vol. 120, pp. 52–60.
Calarco-Gillan, P.D., Siebert, M.C., Hubble, R., Mitchison, T., and Kirschner, M., Centrosome Development in Early Mouse Embryos as Defined by an Autoantibody against Pericentriolar Material, Cell, 1983, vol. 35, pp. 621–629.
Castedo, M., Perfettini, J.L., Roumier, T., Andreau, K., Medema, R., and Kroemer, G., Cell Death by Mitotic Catastrophe: a Molecular Definition, Oncogene, 2004, vol. 23, pp. 2825–2837.
Dodson, H., Wheatley, S.P., and Morrison, C.G., Involvement of Centrosome Amplification in Radiation-Induced Mitotic Catastrophe, Cell Cycle, 2007, vol. 6, pp. 364–370.
Duensing, S. and Münger, K., Human Papillomaviruses and Centrosome Duplication Errors: Modeling the Origins of Genomic Instability, Oncogene, 2002, vol. 21, pp. 6241–6248.
Gadde, S. and Heald, R., Mechanisms and Molecules of the Mitotic Spindle, Curr. Biol., 2004, vol. 14, pp. 797–805.
Gundersen, G.G. and Bulinski, J.C., Distinct Population of Microtubules: Tyrosinated and Nontyrosinated Alpha Tubulin are Distributed Differently in Vivo, Cell. 1984, vol. 38, pp. 779–789.
Illidge, T.M., Cragg, M.S., Fringes, B., Olive, P., and Erenpreisa, J.A., Polyploid Giant Cells Provide a Survival Mechanism for p53 Mutant Cells after DNA Damage, Cell Biol. Int., 2000, vol. 24, pp. 621–633.
Johnson, P.A., Clements, P., Hudson, K., and Caldecott, K.W., A Mitotic Spindle Requirement for DNA Damage-Induced Apoptosis in CHO Cells, Cancer Res., 1999, vol. 59, pp. 2696–2700.
Joshi, H.C., Palacios, M.J., McNamara, L., and Cleveland, D.W., γ-Tubulin Is a Centrosomal Protein Required for Cell Cycle-Dependent Microtubule Nucleation, Nature, 1992, vol. 356, pp. 80–83.
Khodjakov, A. and Rieder, C.L., The Sudden Recruitment of γ-Tubulin to the Centrosome at the Onset of Mitosis and its Dynamic Exchange throughout the Cell Cycle, Do not Require Microtubules, J. Cell Biol., 1999, vol. 146, pp. 585–596.
Komarova, Yu.A., Ryabov, E.V., Alieva, I.B., Uzbekov, R.E., Uzbekova, S.V., and Vorobjev, I.A., Polyclonal Antibodies against Human Gamma-Tubulin Stain Centrioles in Mammalian Cells from Different Tissues, Membr. Cell. Biol., 1997, vol. 10, pp. 503–513.
Lafanechere, L., Courtay-Cahen, C., Kawakami, T., Jacrot, M., Rudiger, M., Wehland, J., Job, D., and Margolis, R.L., Suppression of Tubulin Tyrosine Ligase During Tumor Growth, J. Cell Sci., 1998, vol. 111, pp. 171–181.
Levis, A.G. and Marin, G., Induction of Multipolar Spindle by X-ray in Mammalian Cells in Vitro, Exp Cell Res., 1963, vol. 31, pp. 448–451.
Lingle, W.L., Lutz, W.H., Ingle, J.N., Maihle, N.J., and Salisbury, J.L., Centrosome Hypertrophy in Human Breast Tumors: Implications for Genomic Stability and Cell Polarity, Proc. Natl. Acad. Sci. USA, 1998, vol. 95, pp. 2950–2955.
Lock, R.B. and Ross, W.E., Inhibition of p34cdc2 Kinase Activity by Etoposide or Irradiation as a Mechanism of G2 Arrest in CHO Cells, Cancer Res., 1998, vol. 50, pp. 3761–3766.
Mansilla, S., Bataller, M., and Portugal, J., Mitotic Catastrophe as a Consequence of Chemotherapy, Anticancer Agents Med. Chem., 2006, vol. 6, pp. 589–602.
Onishchenko, G.E., Tsentriolyarnyi i tsentrosomnyi tsikly pri differentsirovke i patologii (Centriolar and Centrosomal Cycles in Differentiation and Pathology), Moscow: Nauka, 1993.
Pan, H., Zhou, F., and Gao, S.J., Kaposi’s Sarcoma-Associated Herpesvirus Induction of Chromosome Instability in Primary Human Endothelial Cells, Cancer Res., 2004, vol. 64, pp. 4064–4068.
Pittman, S., Geyp, M., Fraser, M., Ellem, K., Peaston, A., and Ireland, C., Multiple Centrosomal Microtubule Organising Centres and Increased Microtubule Stability are Early Features of VP-16-Induced Apoptosis in CCRF-CEM Cells, Leuk. Res., 1997, vol. 21, pp. 491–499.
Quintyne, N.J., Reing, J.E., Hoffelder, D.R., Gollin, S.M., and Saunders, W.S., Spindle Multipolarity is Prevented by Centrosomal Clustering, Science, 2005, vol. 307, pp. 127–129.
Rello-Varona, S., Gámez, A., Moreno, V., Stockert, J.C., Cristóbal, J., Pacheco, M., Cañete, M., Juarranz, A., and Villanueva, A., Metaphase Arrest and Cell Death Induced by Etoposide on HeLa Cells, Int. J. Biochem. Cell Biol., 2006, vol. 38, pp. 2183–2195.
Ring, D., Hubble, R., and Kirschner, M., Mitosis in a Cell with Multiple Centrioles, J. Cell Biol., 1982, vol. 94, pp. 549–556.
Sato, Ch., Kuriyama, R., and Nishizawa, K., Microtubule—Organizing Centres Abnormal in Number, Structure and Nucleating Activity in X-Irradiation Mammalian Cells, J. Cell Biol., 1983, vol. 96, pp. 776–782.
Sato, N., Mizumoto, K., Nakamura, M., and Tanaka, M., Radiation-Induced Centrosome Overduplication and Multiple Mitotic Spindles in Human Tumor Cells. Exp. Cell Res., 1983, vol. 255, pp. 321–326.
Saunders, W., Centrosomal Amplification and Spindle Multipolarity in Cancer Cells, Semin. Cancer Biol., 2005, vol. 15, pp. 25–32.
Schiebel, E., γ-Tubulin Complexes: Binding to the Centrosome, Regulation and Microtubule Nucleation, Curr. Opin. Cell Biol., 2000, vol. 12, pp. 113–118.
Schulze, E., Asai, D.J., Bulinski, J.C., and Kirschner, M., Post-translational Modification and Microtubule Stability, J. Cell Biol., 1987, vol. 105, pp. 2167–2177.
Vinogradova, T.M., Balashova, E.E., Smirnov, V.N., and Bystrevskaya, V.B., Detection of the Centriole Tyr-or Acet-Tubulin Changes in Endothelial Cells Treated with Thrombin Using Microscopic Immunocytochemistry, Cell Motil. Cytoskelet., 2005, vol. 62, pp. 1–12.
Wang, Q., Hirohashi, Y., Furuuchi, K., Zhao, H., Liu, Q., Zhang, H., Murali, R., Berezov, A., Du, X., Li, B., and Greene, M.I., The Centrosome in Normal and Transformed Cells, DNA Cell Biol., 2004, vol. 23, pp. 475–489.
Watanabe, N., Yamaguchi, T., Akimoto, Y., Rattner, J.B., Hirano, H., and Nakauchi, H., Induction of M-Phase Arrest and Apoptosis after HIV-1 Vpr Expression through Uncoupling of Nuclear and Centrosomal Cycle in HeLa Cells, Exp Cell Res., 2000, vol. 258, pp. 261–269.
Wehland, J. and Weber, K., Turnover of the Carboxy-Terminal Tyrosine of Alpha-Tubulin and Means of Reaching Elevated Levels of Detyrosination in Living Cells, J. Cell Sci., 1987, vol. 88, pp. 185–203.
Wiese, C. and Zheng, Y., Microtubule Nucleation: Gamma-Tubulin and Beyond, J. Cell Sci., 2006, vol. 119, pp. 4143–4153.
Wolf, K.W. and Spanel-Borowski, K., Acetylation of α-Tubulin in Different Bovine Cell Types: Implications for Microtubule Dynamics in Interphase and Mitosis, Cell Biol. Int., 1995, vol. 19, pp. 43–52.
Yih, L.H., Tseng, Y.Y., Wu, Y.C., and Lee, T.C., Induction of Centrosome Amplification during Arsenite-Induced Mitotic Arrest in CGL-2 Cells, Cancer Res., 2006, vol. 66, pp. 2098–2106.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.E. Balashova, S.S. Ryaskina, T.M. Vinogradova, V.B. Bystrevskaya, 2008, published in Tsitologiya, Vol. 50, No. 3, 2008.
Rights and permissions
About this article
Cite this article
Balashova, E.E., Ryaskina, S.S., Vinogradova, T.M. et al. Organization of mitotic apparatus poles in etoposide-treated CHO-K1 cells. Cell Tiss. Biol. 2, 290–299 (2008). https://doi.org/10.1134/S1990519X08030103
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X08030103