Abstract
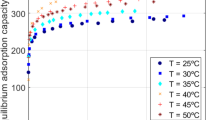
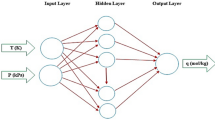
In this study, a three-layer feed-forward back propagation network with Levenberg-Marquardt (LM) learning algorithm was applied to predict adsorption of phenol onto activated carbon (AC). Batch experiments were carried out to obtain experimental data. The neural network was trained considering the amount of adsorbent, initial concentration of phenol, temperature, contact time and pH as input parameters and the final concentration of phenol as a desired parameter. Different transfer functions for hidden and output layers and different number of neurons in a hidden layer were tested to optimize the network structure. An empirical equation for final concentration of phenol was developed by using the weights of optimized network. Accuracy of the developed ANN model was also measured using statistical parameters, such as mean absolute error (MAE), mean square error (MSE), root mean square error (RMSE) and correlation coefficient (R2). Results showed that MAE, MSE, RMSE, and R2 values of the ANN model were 0.1540, 0.0565, 0.2378, and 0.9998, respectively, which indicate high accuracy of the ANN model. In the equilibrium study, predicted results of the ANN model were also compared with experimental data and the results of other conventional isotherm models.
Similar content being viewed by others
References
Canizares, P., Carmona, M., Baraza, O., Delgado, A., and Rodrigo, M.A., Adsorption Equilibrium of Phenol onto Chemically Modified Activated Carbon F400, J. Hazard. Mater., 2006, vol. B131, p. 243.
Mukherjee, S., Kumar, S., Misra, A.K., and Fan, M., Removal of Phenols from Water Environment by Activated Carbon, Bagasse Ash and Wood Charcoal, Chem. Eng. J., 2007, vol. 129, p. 133.
Li, H., Xu, M., Shi, Z., and He, B., Isotherm Analysis of Phenol Adsorption on Polymeric Adsorbents from Nonaqueous Solution, J. Colloid. Interf. Sci., 2004, vol. 271, p. 47.
Qadeer, R. and Rehan, A.H., A Study of the Adsorption of Phenol by Activated Carbon from Aqueous Solutions, Turk. J. Chem., 2002, vol. 26, p. 357.
Srivastava, V.C., Swamy, M.M., Mall, I.D., Prasad, B., and Mishra, I.M., Adsorptive Removal of Phenol by Bagasse Fly Ash and Activated Carbon: Equilibrium, Kinetics and Thermodynamics, Colloid. Surface, A, 2006, vol. 272, p. 89.
Terzyk, A.P., Further Insights into the Role of Carbon Surface Functionalities in the Mechanism of Phenol Adsorption, J. Colloid. Interf. Sci., 2003, vol. 268, p. 301.
Su, F., Lv, L., Hui, T.M., and Zhao, X.S., Phenol Adsorption on Zeolite-Templated Carbons with Different Structural and Surface Properties, Carbon, 2005, vol. 43, p. 1156.
Bahadir, K.K. and Abdurrahman, T., Continuous Electrochemical Treatment of Phenolic Wastewater in a Tubular Reactor, Water. Res., 2003, vol. 37, p. 1505.
Huang, J., Wang, X., Jin, Q., and Wang, Y.Y., Removal of Phenol from Aqueous Solution by Adsorption onto OTMAC-Modified Attapulgite, J. Environ. Manage., 2007, vol. 84, p. 229.
Tomaszewska, M., Mozia, S., and Morawski, W., Removal ofOrganic Matter by Coagulation Enhanced with Adsorption on PAC, Desalination, 2004, vol. 162, p. 79.
Canizares, P., Dominguez, J.A., Rodrigo, M.A., Villasenor, J., and Rodriguez, J., Effect of the Current Intensity in the Electrochemical Oxidation of Aqueous Phenol Wastes at an Activated Carbon and Steel Electrode, Ind. Eng. Chem. Res., 1999, vol. 38, p. 3779.
Jiang, H., Fang, Y., Fu, Y., and Guo, Q.X., Studies on the Extraction of Phenol in Wastewater, J. Hazard. Mater., 2003, vol. 101, p. 179.
Kumar, A., Kumar, Sh., Kumar, S., and Gupta, D.V., Adsorption of Phenol and 4-Nitrophenol on Granular Activated Carbon in Basal Salt Medium: Equilibriumand Kinetics, J. Hazard. Mater., 2007, vol. 147, p. 155.
Canizares, P., Martinez, F., Garcia-Gomez, J., and Rodrigo, M.A., Combined Electrooxidation and Assisted Electrochemical Coagulation of Aqueous Phenol Wastes, J. Appl. Electrochem., 2002, vol. 32, p. 1241.
Kujawski, W., Warszawski, A., Ratajczak, W., Porlmagebski, T., Capalmegea, W., and Ostrowska, I., Application of Pervaporation and Adsorption to the Phenol Removal from Wastewater, Sep. Purif. Technol., 2004, vol. 40, p. 123.
Mustafa, A.I., Alam, Md.S., Amin, Md.M., Bahadur, N.M., and Habib, Md.A., Phenol Removal from Aqueous System by Jute Stick, Pak, J. Anal. Environ. Chem., 2008, vol. 9, p. 92.
Zhang, W.M., Zhang, Q.J., Pan, B.C. Lv, L., Pan, B.J., Xu, Z.W., Zhang, Q.X., Zhao, X.S., Du, W., and Zhang, Q.R., Modeling Synergistic Adsorption of Phenol/Aniline Mixtures in the Aqueous Phase onto Porous Polymer Adsorbents, J. Colloid. Interf. Sci., 2007, vol. 306, p. 216.
Lazarova, Z. and Boyadzhieva, S., Treatment of Phenol-Containing Aqueous Solutions by Membrane-Based Solvent Extraction in Coupled Ultrafiltration Modules, Chem. Eng. J., 2004, vol. 100, p. 129.
Sulaymon, A.H. and Ahmed, K.W., Competitive Adsorption of Furfural and Phenolic Compounds onto Activated Carbon in Fixed Bed Column, Environ. Sci. Technol., 2008, vol. 42, p. 392.
Ania, C.O., Parra, J.B., and Pis, J.J., Effect of Texture and Surface Chemistry on Adsorptive Capacities of Activated Carbons for Phenolic Compounds Removal, Fuel. Process. Technol., 2002, vol. 77, p. 337.
Liao, Q., Sun, J., and Gao, L., The Adsorption of Resorcinol from Water Using Multiwalled Carbon Nanotubes, Colloid. Surface, A, 2008, vol. 312, p. 160.
Ahmaruzzaman, M. and Sharma, D.K., Adsorption of Phenols from Wastewater, Colloid Interface Sci., 2005, vol. 287, p. 14.
Gupta, V.K., Ali, I., and Saini, V.K., Removal of Chlorophenols from Wastewater Using Red Mud: An Aluminum Industry Waste, Environ. Sci. Technol., 2004, vol. 38, p. 4012.
Otero, M., Rozada, F., Calvo, L.F., Garcia, A.I., and Moran, A., Elimination of Organic Water Pollutants Using Adsorbents Obtained from Sewage Sludge, Dyes. Pigments, 2003, vol. no. 57, p. 55.
Batabyal, D., Sahu, A., and Chaudhuri, S.K., Kinetics and Mechanism of Removal of 2,4-Dimethyl Phenol from Aqueous Solutions with Coal Fly Ash, Separ. Technol., 1995, vol. 5, p. 179.
Nayak, P.S. and Singh, B.K., Removal of Phenol from Aqueous Solutions by Sorption on Low Cost Clay, Desalination, 2007, vol. 207, p. 71.
Adak, A., Pal, A., and Bandyopadhyay, M., Removal of Phenol from Water Environment by Surfactant-Modified Alumina through Adsolubilization, Colloid. Surface, A, 2006, vol. 277, p. 63.
Dutta, S., Parsons, S.A., Bhattacharjee, Ch., Bandhyopadhyay, S., and Datta, S., Development of an Artificial Neural Network Model for Adsorption and Photocatalysis of Reactive Dye on TiO2 Surface, Expert. Syst. Appl., 2010, vol. 37, p. 8634.
Langmuir, I., The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum, J. Am. Chem. Soc., 1918, vol. 40, p. 1361.
Freundlich, H., Over the Adsorption in Solution, J. Phys. Chem., 1906, vol. 57, p. 385.
Yetilmezsoy, K., and Demirel, S., Artificial Neural Network (ANN) Approach for Modeling of Pb(II) Adsorption from Aqueous Solution by Antep Pistachio (Pistacia Vera L.) Shells, J. Hazard. Mater., 2008, vol. 153, p. 1288.
Cojocaru, C., Macoveanu, M., and Cretescu, I., Peat-Based Sorbents for the Removal of Oil Spills from Water Surface: Application of Artificial Neural Network Modeling, Colloid. Surface, A, 2011, vol. 384, p. 675.
Aghav, R.M., Kumar, S., and Mukherjee, N., Artificial Neural Network Modeling in Competitive Adsorption of Phenol and Resorcinol from Water Environment Using Some Carbonaceous Adsorbents, J. Hazard. Mater., 2011, vol. 188, p. 67.
Mousavi Dehghani, S.A., Vafaie Sefti, M., Ameri, A., and Shojai Kaveh, N., Minimum Miscibility Pressure Prediction Based on a Hybrid Neural Genetic Algorithm, Chem. Eng. Res. Des., 2008, vol. 86, p. 173.
Sozen, A., Arcaklioglu, E., and Ozalp, M., Formulation Based on Artificial Neural Network of Thermodynamic Properties of Ozone Friendly Refrigerant/Absorbent Couples, Appl. Therm. Eng., 2005, vol. 25, p. 1808.
Maier, H.R. and Dandy, G.C., Neural Networks for the Prediction and Forecasting of Water Resources Variables: A Review of Modeling. Issues and Applications, Environ. Modell. Softw., 2000, vol. 15, p. 101.
Saemi, M., Ahmadi, M., and Yazdian Varjani, A., Design of Neural Networks Using Genetic Algorithm for the Permeability Estimation of the Reservoir, J. Petrol. Sci. Eng., 2007, vol. 59, p. 97.
Senturka, H.B., Ozdesa, D., Gundogdua, A., Durana, C., and Soylak, M., Removal of Phenol from Aqueous Solutions by Adsorption onto Organomodified Tirebolu Bentonite: Equilibrium, Kinetic and Thermodynamic Study, J. Hazard. Mater., 2009, vol. 172, p. 353.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shahryari, Z., Sharifi, A. & Mohebbi, A. Artificial neural network (ANN) approach for modeling and formulation of phenol adsorption onto activated carbon. J. Engin. Thermophys. 22, 322–336 (2013). https://doi.org/10.1134/S181023281304005X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S181023281304005X