Abstract
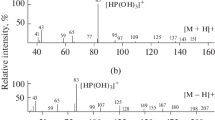
The molecular-mass compositions of the products of partial acidohydrolytic polycondensation that are formed during the interaction of MeSi(OMe)3 with CH3COOH have been studied via MALDI mass spectrometry. It has been shown that, depending on the molar ratio of MeSi(OMe)3 and CH3COOH (m/n), a wide range of oligomethylmethoxysiloxanes with the average composition [MeSiO n/m (OMe)(3 − 2n/m)] p are formed. The analysis of molecular masses and 29Si NMR spectra of the products has revealed various types of molecular structures, which change from linear, branched, and cyclic to polycyclic clusters with condensed cycles after a change in the degree of polycondensation α = 100 × [2n/3m] from 66.67 to 83.33%. At a degree of polycondensation of α = 86.67% or higher, the polycyclic clusters form a spatially crosslinked structure (gel).
Similar content being viewed by others
References
T. A. Tereshenko, Polym. Sci., Ser. B 50, 249 (2008).
T. A. Tereshenko, A. V. Shevchuk, V. V. Shevchenko, S. V. Snigir, and V. A. Pokrovskii, Polym. Sci., Ser. A 48, 1248 (2006).
D. R. Bujalski, H. Chen, R. E. Tecklenburg, E. S. Moyer, G. A. Zank, and K. Su, Macromolecules 36, 180 (2003).
V. V. Kireev, V. F. Posokhova, I. B. Sokol’skaya, V. P. Chuev, V. A. Dyatlov, and S. N. Filatov, Polym. Sci., Ser. B 50, 101 (2008).
V. F. Posokhova, Candidate’s Dissertation in Chemistry (Moscow, 2008).
R. S. Borisov, N. Yu. Polovkov, V. G. Zaikin, and S. N. Filatov, Mass-Spektroskopiya 5, 1 (2008).
E. V. Egorova, N. G. Vasilenko, N. V. Demchenko, E. A. Tatarinova, and A. M. Muzafarov, Dokl. Chem. 424, 15 (2009).
E. V. Parshina, N. G. Vasilenko, N. A. Tebeneva, N. V. Demchenko, and A. M. Muzafarov, in Proceedings of XII Andrianov Conference “Organosiloxane Compounds. Synthesis, Properties, Application”, 2005, Vol. 2, p. 18.
Pat. Appl. No. 2006113775104(014970), Russia (2006), Byull. Izobret., 2007, p. 32.
M. J. Tsai, J. Non-Cryst. Solids 298, 116 (2002).
G. De Karmakar and D. Ganguli, J. Mater. Chem. 10, 2289 (2000).
S. J. Sivananda, J. Am. Ceram. Soc. 70, C298 (1987).
F. J. Feher and T. A. Budzichowski, Org. Chem. 373, 153 (1989).
A. M. Muzafarov, N. G. Vasilenko, E. A. Tatarinova, G. M. Ignat’eva, V. M. Myakushev, M. A. Obrezkova, I. B. Meshkov, N. V. Voronina, and O. V. Novozhilov, Polym. Sci., Ser. C 53, 48 (2011).
A. G. Ivanov, V. M. Kopylov, V. L. Ivanova, V. A. Kovyazin, I. B. Sokol’skaya, and I. I. Khazanov, Zh. Org. Khim. 82, 69 (2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.G. Ivanov, V.M. Kopylov, V.V. Kireev, R.S. Borisov, T.I. Fedotova, Yu.V. Bilichenko, 2014, published in Russian in Vysokomolekulyarnye Soedineniya, Ser. B, 2014, Vol. 56, No. 1, pp. 54–59.
Rights and permissions
About this article
Cite this article
Ivanov, A.G., Kopylov, V.M., Kireev, V.V. et al. A MALDI mass spectrometry investigation of the compositions of the products of the partial acidolysis of MeSi(OMe)3 . Polym. Sci. Ser. B 56, 49–54 (2014). https://doi.org/10.1134/S1560090414010035
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1560090414010035