Abstract
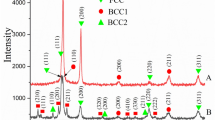
The conditions for preparation of Ce0.9Y0.1O2 (CYO) oxide coatings on La0.8Sr0.2MnO3 (LSM) ceramic substrates by screen printing were investigated. The CYO compound was synthesized by the pyrolysis of polymer-salt composites with the aim of producing submicron powders with a uniform size distribution. Transmission electron microscopy of the microstructure of the CYO compound synthesized with ethylene glycol revealed that the synthesis product consists of ultrafine crystalline particles with an average size of 5–15 nm. The use of CYO nanopowders made it possible to prepare rather dense single-layer coatings on LSM substrates. It was demonstrated that annealing of the coatings at high temperatures leads to the recrystallization and coarsening of particles.
Similar content being viewed by others
References
Ohno, Y., Nagata, S., and Sato, H., Effect of Electrode Materials on the Properties of High-Temperature Solid Electrolyte Fuel Cells, Solid State Ionics, 1981, vols. 3–4, pp. 439–442.
Yamamoto, O., Takeda, Y., Kanno, R., and Noda, M., Perovskite-Type Oxides as Oxygen Electrodes for High Temperature Oxide Fuel Cells, Solid State Ionics, 1987, vol. 22, pp. 241–246.
Kuščer, D., Holc, J., Hrovat, M., Bernik, S., Smardžija, Z., and Kolar, D., Interactions between a Thick Film LaMnO3 Cathode and YSZ SOFC Electrolyte during High Temperature Ageing, Solid State Ionics, 1995, vol. 78, pp. 79–85.
Kostogloudis, G.Ch., Tsiniarakis, G., and Ftikos, Ch., Chemical Reactivity of Perovskite Oxide SOFC Cathodes and Yttria Stabilized Zirconia, Solid State Ionics, 2000, vol. 135, pp. 529–535.
Mori, M., Abe, T., Itoh, H., Yamamoto, O., Shen, G.Q., Takeda, Y., and Imanishi, N., Reaction Mechanism between Lanthanum Manganite and Yttria Doped Cubic Zirconia, Solid State Ionics, 1999, vol. 123, pp. 113–119.
Lee, H.Y. and Oh, S.M., Origin of Cathodic Degradation and New Phase Formation at the La0.9Sr0.1MnO3/YSZ Interface, Solid State Ionics, 1996, vol. 90, pp. 133–140.
Mitterdorfer, A. and Gauckler, L.J., La2Zr2O7 Formation and Oxygen Reduction Kinetics of the La0.85Sr0.15MnyO3, O2(g)|YSZ System, Solid State Ionics, 1998, vol. 111, pp. 185–218.
Simner, S.P., Bonnett, J.F., Canfield, N.L., Meinhardt, K.D., Shelton, J.P., Sprenkle, V.L., and Stevenson, J.W., Development of Lanthanum Ferrite SOFC Cathodes, J. Power Sources, 2003, vol. 113, pp. 1–10.
Mai, A., Haanappel, V.A.C., Uhlenbruck, S., Tietz, F., and Stöver, D., Ferrite-Based Perovskites as Cathode Materials for Anode-Supported Solid Oxide Fuel Cells: Part I. Variation of Composition, Solid State Ionics, 2005, vol. 176, pp. 1341–1350.
Schäfer, W., Koch, A., Herold-Schmidt, U., and Stolten, D., Materials, Interfaces and Production Techniques for Planar Solid Oxide Fuel Cells, Solid State Ionics, 1996, vols. 86–88, pp. 1235–1239.
Vidanov, A.M., Grinenko, E.Yu., Pleshko, Yu.K., Ponomarev, S.V., and Sotnikova, M.N., Composite Pastes for Screen Printing of High-Temperature Superconducting Thick Films, Vysokotemp. Sverkhprovodn., 1989, no. 3, pp. 44–50.
Chick, L.A., Pederson, L.R., Maupin, G.D., Bates, J.L., Thomas, L.E., and Exarhos, G.J., Glycine-Nitrate Combustion Synthesis of Oxide Ceramic Powders, Mater. Lett., 1990, vol. 10, pp. 6–12.
Shankar, K.Sh. and Raychaudhuri, A.K., Low-Temperature Polymer Precursor-Based Synthesis of Nanocrystalline Particles of Lanthanum Calcium Manganese Oxide (La0.67C0.33MnO3) with Enhanced Ferromagnetic Transition Temperature, J. Mater. Res., 2006, vol. 21, no. 1, pp. 27–33.
Park, H.-B., Kweon, H., and Kim, K., Preparation of La1 − x SrxMnO3 Powders by Combustion of Poly(ethylene glycol)-Metal Nitrate Gel Precursors, J. Mater. Sci., 1997, no. 32, pp. 57–65.
Segal, D., Chemical Synthesis of Ceramic Materials, J. Mater. Chem., 1997, vol. 7, no. (8), pp. 1297–1305.
Deshpande, K., Mukasyan, A., and Varma, A., Aqueous Combustion Synthesis of Strontium-Doped Lanthanum Chromite Ceramics, J. Am. Ceram. Soc., 2003, vol. 86, no. 7, pp. 1149–1154.
Zhang, Y., Haung, X., Lu, Zh., Ge, X., Xu, J., Xin, X., Sha, X., and Su, W., Effect of Starting Powder on Screen-Printed YSZ Films Used as Electrolyte in SOFCs, Solid State Ionics, 2006, vol. 177, pp. 281–287.
Zhang, Y.W., Yang, Y., Jin, S., Liao, C.S., and Yan, C.H., Doping Effect on the Grain Size and Microstrain in the Sol-Gel-Derived Rare Earth Stabilized Zirconia Nanocrystalline Thin Films, J. Mater. Sci. Lett., 2002, vol. 21, pp. 943–946.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © Yu.A. Bakhteeva, E.V. Shalaeva, I.A. Leonidov, V.L. Kozhevnikov, 2008, published in Fizika i Khimiya Stekla.
Rights and permissions
About this article
Cite this article
Bakhteeva, Y.A., Shalaeva, E.V., Leonidov, I.A. et al. Investigation of the conditions for synthesis of Ce0.9Y0.1O2 dense coatings. Glass Phys Chem 34, 485–491 (2008). https://doi.org/10.1134/S1087659608040172
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659608040172