Abstract
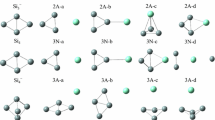
The stabilization of the structure of Si73 clusters that are surrounded by 60 hydrogen atoms and subjected to seventeenfold stepwise heating from 35 to 1560 K (in steps of ∼90 K) is investigated using the molecular dynamics method. The analysis is performed for clusters of three types, i.e., a particle assembled from an icosahedron and a fullerene, a nanocrystal, and a particle with a random atomic packing. In all cases, an increase in the temperature in the course of heating is accompanied by evaporation of a Si atom from the clusters and an increase in the size of silicon particles. The temperature of detachment of Si atoms from clusters is lowest for the cluster with a random atomic packing and highest for the nanocrystal. The nanoassembled particle has the most stable number (close to four) of Si-Si bonds per atom over the entire temperature range 35 ≤ T ≤ 1560 K. For each type of Si73 clusters, the mean length of the Si-Si bond decreases with an increase in the temperature. According to the radial distribution functions, the Si73 clusters have different structures even at the temperature T = 1560 K. The distributions of bond angles reflect the presence of fourfold symmetry elements in the nanoassembled cluster and the nanocrystal. The relative depth of “penetration” of hydrogen atoms into the cluster is largest for the nanocrystal and smallest for the nanoassembled nanoparticle. The largest number of hydrogen atoms is “adsorbed” on the particle with a random atomic packing.
Similar content being viewed by others
References
Hawa, T. and Zachariah, M.R., Molecular Dynamics Study of Particle-Particle Collisions between Hydrogen-Passivated Silicon Nanoparticles, Phys. Rev. B: Condens. Matter, 2004, vol. 69, p. 035417 (1–9).
Ehrman, S.H., Friedlander, S.K., and Zachariah, M.R., Characteristics of SiO2/TiO2 Nanocomposite Particles Formed in a Premixed Flat Flame, J. Aerosol Sci., 1998, vol. 29, nos. 5–6, pp. 687–706.
Windeler, R.S., Lehtinen, K.E.J., and Friedlander, S.K., Production of Nanometer-Sized Metal Oxide Particles by Gas Phase Reaction in a Free Jet. I: Experimental System and Results, Aerosol Sci. Technol., 1997, vol. 27, no. 2, pp. 174–190.
Biswas, P., Yang, G., and Zachariah, M.R., In Situ Processing of Ferroelectric Materials from Lead Waste Streams by Injection of Gas Phase Titanium Precursors: Laser Induced Fluorescence and X-ray Diffraction Measurements, Combust. Sci. Technol., 1998, vol. 134, nos. 1–6, pp. 183–200.
Frenkel, Ya.I., Kineticheskaya teoriya zhidkostei (Kinetic Theory of Liquids), Leningrad: Nauka, 1975 [in Russian].
Hawa, T. and Zachariah, M.R., Internal Pressure and Surface Tension of Bare and Hydrogen Coated Silicon Nanoparticles, J. Chem. Phys., 2004, vol. 121, no. 18, pp. 9043–9049.
Zhang, R.Q., Costa, J., and Bertran, E., Role of Structural Saturation and Geometry in the Luminescence of Silicon-Based Nanostructured Materials, Phys. Rev. B: Condens. Matter, 1996, vol. 53, no. 12, pp. 7847–7850.
Delley, B. and Steigmeier, E.F., Size Dependence of Band Gaps in Silicon Nanostructures, Appl. Phys. Lett., 1995, vol. 67, no. 16, pp. 2370–2372.
Filonov, A.B., Kholod, A.N., Borisenko, V.E., et al., Oxygen Effect on Optical Properties of Nanosize Silicon Clusters, Phys. Rev. B: Condens. Matter, 1998, vol. 57, no. 3, pp. 1394–1397.
Yu, B. and Meyyappan, M., Nanotechnology: Role in Emerging Nanoelectronics, Solid-State Electron., 2006, vol. 50, pp. 536–544.
Xiao, Zh., et al., A Silicon-Based Fuel Cell Micro Power System Using a Microfabrication Technique, J. Micromech. Eng., 2006, vol. 10, no. 16, pp. 2014–2020.
Wise, K.D., Integrated Sensors, MEMS, and Microsystems: Reflection on a Fantastic Voyage, Sens. Actuators, A, 2007, vol. 136, pp. 39–50.
Polukhin, V.A., Modelirovanie nanostruktury i prekursornykh sostoyanii (Simulation of Nanostructures and Precursor States), Yekaterinburg: Ural. Otd. Ross. Akad. Nauk, 2004.
Tersoff, J., New Empirical Approach for the Structural and Energy of Covalent Systems, Phys. Rev. B: Condens. Matter, 1988, vol. 37, no. 10, pp. 6991–7000.
Tersoff, J., Modeling Solid-State Chemistry: Interatomic Potentials for Multicomponent Systems, Phys. Rev. B: Condens. Matter, 1989, vol. 39, no. 8, pp. 5566–5568.
Mousseau, N. and Lewis, L.J., Dynamical Models of Hydrogenated Amorphous Silicon, Phys. Rev. B: Condens. Matter, 1991, vol. 43, pp. 9810–9817.
Kwon, I., Biswas, R., and Soukoulis, C.M., Molecular-Dynamics Simulations of Defect Formation in Hydrogenated Amorphous Silicon, Phys. Rev. B: Condens. Matter, 1992, vol. 45, no. 7, pp. 3332–3339.
Biswas, P. and Hamman, D.R., New Classical Models for Silicon Structural Energies, Phys. Rev. B: Condens. Matter, 1987, vol. 36, no. 12, pp. 6434–6445.
Yin, M.T. and Cohen, M.L., Theory of Static Structural Properties, Crystal Stability, and Phase Transformations: Application to Si and Ge, Phys. Rev. B: Condens. Matter, 1982, vol. 26, no. 10, pp. 5668–5687.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.E. Galashev, I.A. Izmodenov, 2008, published in Fizika i Khimiya Stekla.
Rights and permissions
About this article
Cite this article
Galashev, A.E., Izmodenov, I.A. Computer investigation of the structure of Si73 clusters surrounded by hydrogen. Glass Phys Chem 34, 173–181 (2008). https://doi.org/10.1134/S1087659608020107
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659608020107