Abstract
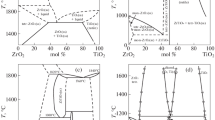
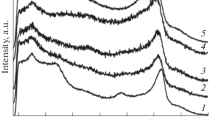
Amorphous nanomaterials in the ZrO2-CeO2 system are synthesized. The aging and crystallization processes in a complex zirconium-cerium hydrogel are investigated. Powders with a crystal size of 45–100 nm are prepared. Samples having a porosity of less than 2.2% are produced from the powders. It is demonstrated that tetragonal zirconia-based solid solutions Zr1−x CexO2−δ sintered at a temperature of 1500°C possess ionic conductivity in air and in noble gasses. The assumptions are made regarding the mechanisms of ionic (surface anionic) and electronic (hopping) conduction in samples of the 88ZrO2 · 12CeO2 (mol %) composition. The solid solutions prepared can be used at a temperature of 720°C as high-strength inexpensive solid electrolytes.
Similar content being viewed by others
References
Glushkova, V.B., Podzorova, L.I., Popov, V.P., and Tikhonov, P.A., Electric Transport Properties and the Size Effect in ZrO2-Based Ceramic Materials, Tezisy dokladov VIII Mezhdunarodnogo simpoziuma “Poryadok, besporyadok i svoistva oksidov” (Abstracts of Papers of the VIII International Symposium “Order, Disorder, and Oxide Properties”), Sochi, 2005, p. 55 [in Russian].
Pal’guev, S.F. and Volchenkova, Z.S., Electrical Conductivity and Transport Numbers of Ions in the CeO2-ZrO2 System, Zh. Fiz. Khim., 1960, vol. 34, no. 2, pp. 452–455.
Larry, L., Fehrenbacher, N., and Tallan, M., Electrical Property of CeO2-ZrO2, Ceram. Bull., 1969, vol. 48, no. 4, p. 409.
Podzorova, L.I., Il’icheva, A.A., Mikhailina, N.A., Shevchenko, V.Ya., et al., Investigation of Homogeneous Precipitation of Zirconia Stabilized by Ceria, Ogneupory, 1995, no. 6, pp. 2–5.
Il’icheva, A.A., Olenin, A.Yu., Podzorova, L.I., Shevchenko, V.Ya., et al., Surfactant Effects on the Aggregation and Structure of Stabilized Zirconia Prepared by Sol-Gel Processing, Neorg. Mater., 1996, vol. 32, no. 7, pp. 833–837 [Inorg. Mater. (Engl. transl.), 1996, vol. 32, no. 7, pp. 736–740].
Podzorova, L.I., Il’icheva, A.A., Mikhailina, N.A., Shevchenko, V.Ya., et al., Effect of Synthesis Conditions on the Phase Composition of ZrO2-CeO2-Al2O3 Sol-Gel Powders, Neorg. Mater., 2001, vol. 37, no. 1, pp. 60–66 [Inorg. Mater. (Engl. transl.), 2001, vol. 37, no. 1, pp. 51–57].
Shevchenko, V.Ya., Glushkova, V.B., Panova, T.I., Podzorova, L.I., et al., Preparation of Ultrafine Tetragonal ZrO2-CeO2 Solid Solutions, Neorg. Mater., 2001, vol. 37, no. 7, pp. 821–827 [Inorg. Mater. (Engl. transl.), 2001, vol. 37, no. 7, pp. 692–697].
Author information
Authors and Affiliations
Additional information
Original Russian Text © V.B. Glushkova, V.P. Popov, P.A. Tikhonov, L.I. Podzorova, A.A. Il’icheva, 2006, published in Fizika i Khimiya Stekla.
Rights and permissions
About this article
Cite this article
Glushkova, V.B., Popov, V.P., Tikhonov, P.A. et al. Electrtic transport properties and the size effect in ZrO2-based ceramic materials. Glass Phys Chem 32, 587–590 (2006). https://doi.org/10.1134/S1087659606050142
Received:
Issue Date:
DOI: https://doi.org/10.1134/S1087659606050142