Abstract
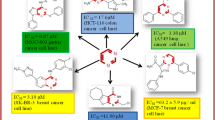
A novel series of 1,2,3-triazolylthiazolidinedione analogs have been synthesized by the condensation of the corresponding 1-aryl-1H-1,2,3-triazole-4-carbaldehydes with thiazolidine-2,4-dione in the presence of KOH. The title compounds were evaluated for their in vitro anticancer activity using MTT assay against four cancer cell lines: A549 (lung), HT-29 (colon), MCF-7 (breast), and A375 (melanoma). Most compounds displayed good anticancer activity, but hydroxy- and nitro-substituted derivatives showed higher activity than the others.


Similar content being viewed by others
REFERENCES
Arshad, M., Shoeb Khan, M., Asghar Nami, S.A., and Ahmad, D., Russ. J. Gen. Chem., 2018, vol. 88, p. 2154. https://doi.org/10.1134/S1070363218100213
Terzioglu, N., Karali, N., Gursoy, A., Pannecouque, C., Leysen, P., Paeshuyse, J., Neyts, J., and Clercq, E., Arkivoc, 2006, vol. x, p. 109. http://dx.doi.org/10.3998/ark.5550190.0007.113
Sohda, T., Mizuno, K., Tawada, H., Sugiyama, Y., Fugita, T., and Kawamatsu, Y., Chem. Pharm. Bull., 1982, vol. 30, p. 3563. https://doi.org/10.1248/cpb.30.3563
Goel, B., Ram, T., Tyagi, R., Bansal, E., Kumar, A., Mukherjee, D., and Sinha, J.N., Eur. J. Med. Chem., 1999, vol. 34, p. 265. https://doi.org/10.1016/S0223-5234(99)80060-9
Sharma, R.K., Younis, Y., Mugumbate, G., Njoroge, M., Gut, J., Rosenthal, P.J., and Chibale, K., Eur. J. Med. Chem., 2015, vol. 90, p. 507. https://doi.org/10.1016/j.ejmech.2014.11.061
Balzarini, J., Orzeszko, B, Maurin, J.K., and Orzeszko, A., Eur. J. Med. Chem., 2007, vol. 42, p. 993. https://doi.org/10.1016/j.ejmech.2007.01.003
Fathy, U., Gouhar, R.S., Awad, H.M., and Abdel-Aziz, H.A., Russ. J. Gen. Chem., 2017, vol. 87, p. 2951. https://doi.org/10.1134/S1070363217120374
Mizzoni, R.H. and Eisman, P.C., J. Am. Chem. Soc., 1957, vol. 80, p. 3471. https://doi.org/10.1021/ja01546a066
Ottana, R., Maccari, R., Giglio, M., Del Corso, A., Cappiello, M., Mura, U., Cosconati, S., Marinelli, L., Novellino, E., Sartini, S., La Motta, C., and Da Settimo, F., Eur. J. Chem., 2011, vol. 46, p. 2797. https://doi.org/10.1016/j.ejmech.2011.03.068
Ashok, D., Ganesh, A., Vijaya Lakshmi, B., Ravi, S., and Ramesh, B., Org. Commun., 2014, vol. 8, p. 24.
Ashok, D., Ravi, S., Ganesh, A., and Vijaya Lakshmi, B., Med. Chem. Res., 2016, vol. 25, p. 909. https://doi.org/10.1007/s00044-016-1537-7
Ming-Xia, S. and Xian-Qing, D., J. Enz. Inhibition Med. Chem., 2018, vol. 33, p. 1453. https://doi.org/10.1080/14756366.2018.1512597
Kumar, A., Sathish Kumar, B., Sreenivas, E., and Subbaiah, T., Russ. J. Gen. Chem., 2018, vol. 88, p. 587. https://doi.org/10.1134/S1070363218030313
Lazrek, H.B., Taourirte, M., Oulih, T., Barascut, J.L., Imbach, JL., Pannecouque, C., Witrouw, M., and De Clercq, E., Nucleosides Nucleotides Nucleic Acids, 2001, vol. 20, p. 1949. https://doi.org/10.1081/NCN-100108325
Mubarak, H.S., Dnyaneshwar, D.S., Laxman, N.D., Sarkar, F., Kalam Khan, A., Jaiprakash, N.S., and Bapurao, B.S., Med. Chem. Commun., 2015, vol. 6, p. 1104. https://doi.org/10.1039/C5MD00057B
Mounika, P., Aishwarya, M.N.L., Pranabesh, S., Prathima, S., and Niranajan, B.M., Asian J. Pharm. Res., 2017, vol. 7, p. 124. https://doi.org/10.5958/2231-5691.2017.00021.1
Shahnaz, Md., Bhai, P.K., and Bhai, R., J. Drug Deliv. Ther., 2013, vol. 3, p. 96. https://doi.org/10.22270/jddt.v3i6.714
Bahare, R.S. and Kulkarni, V.M., Der Pharma Chem., 2011, vol. 3, p. 164.
Boechat, N., Ferreira, V.F., Ferreira, S.B., de Lourdes, G., Ferreira, M., de, C., da Silva, F., Bastos, M.M., Dos, S., Costa, M., Lourenço, M.C., Pinto, A.C., Krettli, A.U., Aguiar, A.C., Teixeira, B.M., da Silva, N.V., Martins, P.R., Bezerra, F.A., Camilo, A.L., da Silva, G.P., and Costa, C.C., J. Med. Chem., 2011, vol. 54, p. 5988. https://doi.org/10.1021/jm2003624
Aliabadi, A., Shamsa, F., Ostad, S.N., Emami, S., Shafiee, A., Davoodi, J., and Foroumadi, A., Eur. J. Med. Chem., 2010, vol. 11, p. 5384. https://doi.org/10.1016/j.ejmech.2010.08.063
Bilge, B., Serda, K.G., Yagmur, K., Aysen, E.O., and Sevim, A., Bilge Int. J. Sci. Technol. Res., 2019, vol. 3, p. 1. https://doi.org/10.30516/bilgesci.476841
Degorce, S.L., Barlaam, B., Cadogan, E., Dishington, A.P., Ducray, R., Glossop, S.C., Hassall, L.A., Lach, F., Lau, A., Mcguire, T.M., Nowak, T., Ouvry, G., Pike, K.G., and Thomason, A.G., J. Med. Chem., 2016, vol. 59, p. 6281. https://doi.org/10.1021/acs.jmedchem.6b00519
Hassanin, H.M., Serya, R.A.T., Abd Elmoneam, W.R., and Mostafa, M.A., R. Soc. Open Sci., 2018, vol. 5, p. 172407. https://doi.org/10.1098/rsos.172407
ACKNOWLEDGMENTS
The authors are grateful to the Department of Science of Humanities, Vignan’s Foundation for Science Technology and Research (VFSTR) University, for providing laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Supplementary material
Rights and permissions
About this article
Cite this article
Manikala, V.K., Rao, V.M. Synthesis and Anticancer Activity of (E)-5-[(1-Aryl-1H-1,2,3-triazol-4-yl)methylene]thiazolidine-2,4-diones. Russ J Org Chem 56, 863–868 (2020). https://doi.org/10.1134/S1070428020050206
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428020050206