Abstract
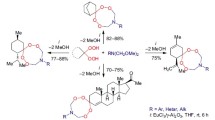
New tetracyclic dispiro-1,2,4-trioxolanes (ozonides) were synthesized by reactions of 5–7-membered alicyclic 1,5-diketones with 30% hydrogen peroxide in diethyl ether or ethanol in the presence of boron trifluoride-diethyl ether complex or a strong mineral acid (HCl, H2SO4, HClO4). Mass spectrometric study of the title compounds under atmospheric pressure chemical ionization revealed specificity of fragment ion decomposition with respect to the ring size, which makes it possible to identify such compounds by their mass spectra.
Similar content being viewed by others
References
Akimova, T.I. and Soldatkina, O.A., RU Patent no. 2578609, 2016; Byull. Izobret, 2016, no. 9.
Kondelikova, J., Kralicek, J., and Kubanek, V., Collect. Czech. Chem. Commun., 1972, vol. 37, p. 263.
Gomes, G.P., Yaremenko, I.A., Radulov, P.S., Novikov, R.A., Chernyshev, V.V., Korlyukov, A.A., Nikishin, G.I., Alabugin, I.V., and Terent’ev, A.O., Angew. Chem., Int. Ed., 2017, vol. 56, p. 4955.
Yaremenko, I.A., Gomes, G.P., Radulov, P.S., Belyakova, Yu.Yu., Vilikotskiy, A.E., Vil’, V.A., Korlyukov, A.A., Nikishin, G.I., Alabugin, I.V., and Terent’ev, A.O., J. Org. Chem., 2018, vol. 83, p. 4402.
Yaremenko, I.A., Terent’ev, A.O., Vil’, V.A., Novikov, R.A., Chernyshev, V.V., Tafeenko, V.A., Levitsky, D.O., Fleury, F., and Nikishin, G.I., Chem. Eur. J., 2014, vol. 20, p. 10160.
Criegee, R. and Lohaus, G., Chem. Ber., 1953, vol. 86, p. 1.
Griesbaum, K., Liu, X., and Dong, Y., Tetrahedron, 1997, vol. 53, p. 5463.
Griesbaum, K., Liu, X., Kassiaris, A., and Scherer, M., Justus Liebigs Ann. Chem., 1997, p. 1381.
Barton, V., Ward, S.A., Chadwick, J., Hill, A., and O’Neill, P.M., J. Med. Chem., 2010, vol. 53, p. 4555.
Dong, Y., Chollet, J., Matile, H., Charman, S.A., Chiu, F.C.K., Charman, W.N., Scorneaux, B., Urwyler, H., Tomas, J.S., Scheurer, C., Snyder, C., Dorn, A., Wang, X., Karle, J.M., Tang, Y., Wittlin, S., Brun, R., and Vennerstrom, J.L., J. Med. Chem., 2005, vol. 48, p. 4953.
Dong, Y., Wittlin, S., Sriraghavan, K., Chollet, J., Charman, S.A., Charman, W.N., Scheurer, C., Urwyler, H., Tomas, J.S., Snyder, C., Creek, D.J., Morizzi, J., Koltun, M., Matile, H., Wang, X., Padmanilayam, M., Tang, Y., Dorn, A., Brun, R., and Vennerstrom, J.L., J. Med. Chem., 2010, vol. 53, p. 481.
Dong, Y., Tang, Y., Chollet, J., Matile, H., Wittlin, S., Charman, S.A., Charman, W.N., Tomas, J.S., Scheurer, C., Snyder, C., Scorneaux, B., Bajpai, S., Alexander, S.A., Wang, X., Padmanilayam, M., Cheruku, S.R., Brun, R., and Vennerstrom, J.L., Bioorg. Med. Chem., 2006, vol. 14, p. 6368.
Stocks, P.A., Bray, P.G., Barton, V.E., Al-Helal, M., Jones, M., Araujo, N.C., Gibbons, P., Ward, S.A., Hughes, R.H., Biagini, G.A., Davies, J., Amewu, R., Mercer, A.E., Ellis, G., and O’Neill, P.M., Angew. Chem., Int. Ed., 2007, vol. 46, p. 6278.
Zhou, L., Alker, A., Ruf, A., Wang, X., Chiu, F.C.K., Morizzi, J., Charman, S.A., Charman, W.N., Scheurer, C., Wittlin, S., Dong, Y., Hunziker, D., and Vennerstrom, J.L., Bioorg. Med. Chem. Lett., 2008. vol. 18, p. 1555.
Butler, A.R. and Yu-Lin Wu, Chem. Soc. Rev., 1992, vol. 21, p. 85.
Opsenica, D.M. and Šolaja, B.A., J. Serb. Chem. Soc., 2009, vol. 74, p. 1155.
Araujo, N.C.P., Barton, V., Jones, M., Stocks, P.A., Ward, S.A., Davies, J., Bray, P.G., Shone, A.E., Cristiano, M.L.S., and O’Neill, P.M., Bioorg. Med. Chem. Lett., 2009, vol. 19, p. 2038.
O’Neill, P.M., Amewu, R.K, Nixon, G.L., El-Garah, F.B., Mungthin, M., Chadwick, J., Shone, A.E., Vivas, L., Lander, H., Barton, V., Muangnoicharoen, S., Bray, P.G., Davies, J., Park, B.K., Wittlin, S., Brun, R., Preschel, M., Zhang, K., and Ward, S.A., Angew. Chem., Int. Ed., 2010, vol. 49, p. 5693.
Sumit, S., Mahajan, S.S., Deu, E., Lauterwasser, E.M.W., Leyva, M.J.L., Ellman, J.A., Bogyo, M., and Renslo, A.R., ChemMedChem, 2011, vol. 6, p. 415.
Henriksen, B., Tysor, J.L., and Lomneth, R., J. Chem. Pharm. Res., 2012, vol. 4, no. 4, p. 2012.
Wang, X., Dong, Y., Wittlin, S., Charman, S.A., Chiu, F.C.K., Chollet, J., Katneni, K., Mannila, J., Morizzi, J., Ryan, E., Scheurer, C., Steuten, J., Tomas J.S., Snyder, C., and Vennerstrom, J.L., J. Med. Chem., 2013, vol. 56, p. 2547.
Cullen, B., Henriksen, B., and Lomneth, R., J. Chem. Pharm. Res., 2014, vol. 6, no. 10, p. 329.
Sun, S., Zhao, Y-Y., and Curtis, J.M., Rapid Commun. Mass Spectrom, 2012, vol. 26, p. 921.
Thomas, M.C., PhD Thesis, University of Wollongong, 2012.
Author information
Authors and Affiliations
Corresponding author
Additional information
Deceased.
Rights and permissions
About this article
Cite this article
Akimova, T.I., Rybin, V.G. & Soldatkina, O.A. New Tetracyclic Spiro-1,2,4-trioxolanes (Ozonides). Synthesis and Mass Spectrometric Study. Russ J Org Chem 55, 101–107 (2019). https://doi.org/10.1134/S1070428019010123
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428019010123