Abstract
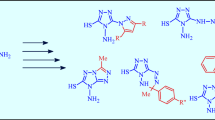
Acylation of benzene and toluene with 5-phenyl- and 5-(p-tolyl)isoxazole-3-carbonyl chlorides gave 5-phenyl(or p-tolyl)isoxazol-3-yl phenyl(or p-tolyl)ketones which were reduced to the corresponding alcohols with sodium tetrahydridoborate in propan-2-ol. Selective reduction of the carboxy group in 4,5-dichloroisothiazole-3-carboxylic acid was achieved by the action of BH3, and the aldehyde group in 4-formyl-2-methoxyphenyl 5-arylisoxazole-3-carboxylates and 4,5-dichloroisothiazole-3-carboxylates was reduced to hydroxymethyl group with sodium triacetoxyhydridoborate in benzene. Acylation of the resulting hydroxymethyl derivatives with 5-arylisoxazole- and 4,5-dichloroisothiazole-3-carbonyl chlorides afforded the corresponding esters containing two azole fragments in their molecules.
Similar content being viewed by others
References
Murugesan, N., Gu, Z., Spergel, S., Young, M., Chen, P., Mathur, A., Leith, L., Hermsmeier, M., Liu, E.C., Zhang, R., Bird, E., Waldron, T., Marino, A., Koplowitz, B., Humphreys, W.G., Chong, S., Morrison, R.A., Webb, M.L., Moreland, S., Trippodo, N., and Barrish, J.C., J. Med. Chem., 2003, vol. 46, p. 125; Gardner, T.S., Wenis, E., and Lee, J., J. Med. Chem., 1959, vol. 2, p. 133; Pinto, P. and Dougados, M., Acta Reumatol. Port., 2006, vol. 31, p. 215; Talley, J.J., Brown, D.L., Carter, J.S., Graneto, M.J., Koboldt, C.M., Masferrer, J.L., Perkins, W.E., Rogers, R.S., Shaffer, A.F., Zhang, Y.Y., Zweifel, B.S., and Seibert, K., J. Med. Chem., 2000, vol. 43, p. 775.
Shailaja, M., Manjula, A., and Vittal Rao, B., Indian J. Chem., Sect. B, 2011, vol. 50, p. 214; Rzeski, W., Ikonomidou, Ch. and Turski, L., Biochem. Pharmacol., 2002, vol. 64, p. 1195; Kamal, A., Bharathi, E.V., Reddy, J.S., Ramaiah, M.J., Dastagiri, D., Reddy, K., Viswanath, A., Reddy, T.L., Shaik, T.B., Pushpavalli, S.N.C.V.L., and Bhadra, M.P., Eur. J. Med. Chem., 2011, vol. 46, p. 691.
Clerici, F., Gelmi, M.L., Pellegrino, S., and Pocar, D., Bioactive Heterocycles III, Khan, M.T.H., Ed., Berlin: Springer, 2007, p. 179.
Beebe, J.S., Jani, J.P., Knauth, E., Goodwin, P., Higdon, C., Rossi, A.M., Emerson, E., Finkelstein, M., Floyd, E., Harriman, S., Atherton, J., Hillerman, S., Soderstrom, C., Kou, K., Gant, T., Noe, M.C., Foster, B., Rastinejad, F., Marx, M.A., Schaeffer, T., Whalen, P.M., and Roberts, W.G., Cancer Res., 2003, vol. 63, p. 7301.
Kulchitsky, V.A., Potkin, V.I., Zubenko, Yu.S., Chernov, A.N., Talabaev, M.V., Demidchik, Yu.E., Petkevich, S.K., Kazbanov, V.V., Gurinovich, T.A., Roeva, M.O., Grigoriev, D.G., Kletskov, A.V., and Kalunov, V.N., Med. Chem., 2012, vol. 8, p. 22.
Potkin, V.I., Dikusar, E.A., and Petkevich, S.K., Dokl. Nats. Akad. Navuk Belarusi, 2008, vol. 52, p. 60.
Potkin, V.I., Gadzhily, R.A., Dikusar, E.A., Petkevich, S.K., Zhukovskaya, N.A., Aliev, A.G., and Nagieva, Sh.F., Russ. J. Org. Chem., 2012, vol. 48, p. 127.
Potkin, V.I., Kletskov, A.V., Petkevich, S.K., Khotyanovich, M.O., Zubenko, Yu.S., and Kul’chitskii, V.A., Russ. J. Org. Chem., 2013, vol. 49, p. 283.
Dikusar, E.A., Nechai, N.I., Potkin, V.I., Kaberdin, R.V., Kozlov, N.G., and Kovganko, N.V., Khim. Prirodn. Soedin., 2003, vol. 39, p. 186; Potkin, V.I., Zubenko, Yu.S., Bykhovetz, A.I., Zolotar, R.M., and Goncharuk, V.M., Nat. Prod. Commun., 2009, vol. 4, p. 1205.
Nechai, N.I., Dikusar, E.A., Potkin, V.I., and Kaberdin, R.V., Russ. J. Org. Chem., 2004, vol. 40, p. 1009.
Zapol’skii, V.A., Namyslo, J.C., de Meijere, A., and Kaufmann, D.E., Beilst. J. Org. Chem., 2012, vol. 8, p. 621.
Takhistov, V.V., Prakticheskaya mass-spektrometriya organicheskikh soedinenii (Practical Mass Spectrometry of Organic Compounds), Leningrad: Leningr. Gos. Univ., 1977, p. 265; Takhistov, V.V., Rodin, A.A., and Maksimova, B.N., Usp. Khim., 1991, vol. 60, p. 2143.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.I. Potkin, S.K. Petkevich, A.V. Kletskov, E.A. Dikusar, Yu.S. Zubenko, N.A. Zhukovskaya, V.V. Kazbanov, S.G. Pashkevich, 2013, published in Zhurnal Organicheskoi Khimii, 2013, Vol. 49, No. 10, pp. 1543–1553.
Rights and permissions
About this article
Cite this article
Potkin, V.I., Petkevich, S.K., Kletskov, A.V. et al. Synthesis of functionally substituted isoxazole and isothiazole derivatives. Russ J Org Chem 49, 1523–1533 (2013). https://doi.org/10.1134/S1070428013100205
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428013100205