Abstract
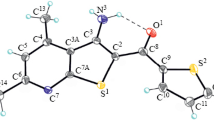
The reaction of N-methyl-N-(diethoxycarbonyl)methyltetrahydropyridinium bromide with dimethyl acetylenedicarboxylate in the presence of triethylamine at room temperature afforded 1,2-dimethyl 1-ethyl 2-[(3-vinyl-1-methyl-3-phenyl-2-ethoxycarbonyl)pyrrolidin-2-yl]-ethene-1,1,2-tricarboxylate in 25% yield. Its structure was proved by XRD analysis. At cooling to −20°C the pyrrolidine yield signifi cantly decreased and 3,4-dimethyl 2,2-diethyl 1-methyl-7-phenyl-1,5,8,9-tetrahydro-2H-azonine-2,2,3,4-tetratcarboxylate was obtained in 31% yield.
Similar content being viewed by others
References
Soldatenkov, A.T., Volkov, S.V., and Soldatova, S.A., Khim. Geterotsikl. Soedin., 2007, p. 613; Gimranova, G.S., Soldatova, S.A., Prokudina, E.G., Soldatenkov, A.T., and Polyanskii, K.B., Zh. Org. Khim., 2008, vol. 44, p. 1416.
Maridass, M., Raju, G., Thangavel, K., Ghanthikumar, S. Ethnobotanical Leafl ets, 2008, vol. 12, p. 954, http://195.178.207.233/PASS/; Gimranova, G.S., Cand Sci. (Chem.) Dissertation, Moscow, 2008.
Mageswaran, S., Ollis, W.D., and Sutherland, I.O., J. Chem. Soc. Perkin, Trans. 1, 1981, p. 1953; Sweeney, J.B., Tavassoli, A., Carter, N.B., and Hayes, J.F., Tetrahedron, 2002, 58, 10113; Soldatova, S.A., Akbulatov, S.V., Gimranova, G.S., Rudakov, Yu.O., Polyanskii, K.B., and Soldatenkov, A.T., Khim. Geterotsikl. Soedin., 2005, p. 790; Soldatova, S.A., Gimranova, G.S., Mamyrbekova, Zh.A., Polyanskii, K.B., Akbulatov, S.V., and Soldatenkov, A.T., Khim. Geterotsikl. Soedin., 2007, p. 1670.
Schmidle, C.J. and Mansfield, R.C. J. Am. Chem. Soc., 1956, vol. 78, p. 425.
Sheldrick, G.M., Acta Cryst., 2008, vol. A64, p. 112.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.T. Soldatenkov, S.A. Soldatova, R.R Suleimanov, K. B. Polyanskii, V.E. Kotsyuba, A.F. Smol’yakov, V.N. Khrustalev, M.Yu. Antipin, 2011, published in Zhurnal Organicheskoi Khimii, 2011, Vol. 47, No. 11, pp. 1700–1703.
Rights and permissions
About this article
Cite this article
Soldatenkov, A.T., Soldatova, S.A., Suleimanov, R.R. et al. Synthesis of pyrrolidine and tetrahydroazonine derivatives from N-[bis(ethoxycarbonyl)methyl]tetrahydropyridinium bromide and methyl acetylenedicarboxylate. Russ J Org Chem 47, 1738–1741 (2011). https://doi.org/10.1134/S1070428011110157
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428011110157