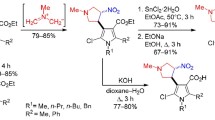
Abstract
The reaction of ethyl 4-formyl-1-phenyl-1H-pyrazole-3-carboxylate with the malonic acid led to the formation (2E)-3-(3-ethoxycarbonyl-1-phenyl-1H-pyrazol-4-yl)propenic acid. In reactions of this acid chloride with 4-amino-5-aryl(hetaryl)-4H-1,2,4-triazole-3-thiols were obtained ethyl 4-[(E)-2-{3-aryl(hetaryl)[1,2,4]triazolo[3,4-b][1,3,4]thiadiazol-6-yl}ethenyl]-1-phenyl-1H-pyrazoe-3-carboxylates, with 5-aryltetrazoles, ethyl 4-[(E)-2-(5-aryl-1,3,4-oxadiazol-2-yl)-ethenyl]-1-phenyl-1H-pyrazole-3-carboxylates, with 1-(2-hydroxy-3,5-dimethylphenyl) followed by the Baker-Venkataraman rearrangement and the cyclization, ethyl 4-[(E)-2-(6,8-dimethyl-4-oxo-4Hchromen-2-yl)ethenyl]-1-phenyl-1H-pyrazole-3-carboxylate.
Similar content being viewed by others
References
Yokoyama, Y., Kurimoto, Y., Saito, Y., Katsurada, M., Okada, I., Osano Y.T., Sasaki, C., Yokoyama, Y., Tukada, H., Adachi, M., Nakamura, S., Murayama, T., Harazono, T., and Kodaira, T., Chem. Lett., 2004, vol. 33, p. 106; Sridhar, R., Perumal, P.T., Tetrahedron., 2005, vol. 61, p. 2465.
Vovk, M.V., Bratenko, M.K., and Chornous, V.O., 4-Funktsional’no zamishcheni pirazoli. Chernivtsi: Prut, 2008, p. 145.
Matiichuk, V.S., Potopnyk, M.A., and Obushak, N.D., Zh. Org. Khim., 2008, vol. 44, p. 1368.
Soliman, F.S.G. and Shafik, R.M., Pharmazie., 1975, vol. 30, p. 436.
Rostom, S.A.F., Shalaby, M.A., and El-Demellawi, M.A., Eur. J. Med. Chem., 2003, vol. 38, p. 959.
Lan, R., Liu, Q., Fan, P., Lin, S., Fernando, S.R., McCallion, D., Pertwee, R., and Makriyannis, A., J. Med. Chem., 1999, vol. 42, p. 769.
Meschler, J.P., Kraichely, D.M., Wilken, G.H., and Howlett, A.C., Biochem. Pharmacol., 2000, vol. 60, p. 1315.
Fougerousse, A., Gonzalez, E., and Brouillard, R., J. Org. Chem., 2000, vol. 65, p. 583; Pinto, D.C.G.A., Silva, A.M.S., Almeida, L.M.P.M., Cavaleiro, J.A.S., and Elguero, J. Eur. J. Org. Chem., 2002, p. 3807; Ganguly, A.K., Kaur, S., Mahata, P.K., Biswas, D., Pramanik, B.N., and Chan, T.M., Tetrahedron Lett., 2005, vol. 46, p. 4119.
Sosnovskikh, V.Ya., Usachev, B.I., and Vorontsov, I.I., Tetrahedron, 2003, vol. 59, p. 2549.
Kanaoka, M., J. Pharm. Soc. Jpn., 1956, vol. 76, p. 1133.
Shaker, R.M., Aly, A.A. Phosph., Sulfur, Silicon. Relat. Elem., 2006, vol. 181, p. 2577.
Obushak, N.D., Pokhodylo, N.T., Krupa, I.I., and Matiichuk, V.S., Zh. Org. Khim., 2007, vol. 43, p. 1227; Matiichuk, V.S., Pokhodilo, N.T., Krupa, I.I., and Obushak, M.D., Ukr. Bioorg. Acta, 2007, vol. 5, p. 3; http://www.bioorganica.org.ua/UBAdenovo/vol_5_1.htm.
Obushak, M.D., Pokhodylo, N.T., Ostapiuk, Yu.V., and Matiychuk, V.S., Phosph., Sulfur, Silicon. Relat. Elem., 2008, vol. 183, p. 141.
Holla, B.S., Shivananda, M.K., Akberali, P.M., Baliga, S., and Safeer, S., Farmaco., 1996, vol. 51, p. 785; Zhang, Q., Pan, J., Zheng, R.-L., and Wang, Q., Pharmazie, 2005, vol. 60, p. 378; Spalinska, K., Foks, H., Kedzia, A., Wierzbowska, M., Kwapisz, E., Gebska, A., and Zilkoiwska-Klinkosz, M., Phosph., Sulfur, Silicon. Relat. Elem., 2006, vol. 181, p. 609; Invidiata, F.P., Furno, G., Simoni, D., Lampronti, I., Musiu, C., Milia, C., Scintu, F., and La Colla, P., Farmaco, 1997, vol. 52, p. 259; Holla, B.S., Poojary, K.N., Rao, B.S., and Shivananda, M.K., Eur. J. Med. Chem., 2002, vol. 37, p. 511.
Huisgen, R., Sauer, J., Sturm, H.J., and Markgraf, J.H., Chem. Ber., 1960, vol. 93, p. 2106; Ollis, W.D. and Ramsden, C.A., Adv. Heterocycl. Chem., 1976, vol. 19, p. 1.
Obushak, N.D., Pokhodylo, N.T., Pidlypnyi, N.I., and Matiichuk, V.S., Zh. Org. Khim., 2008, vol. 44, p. 1544.
Kadaba, P.K., Synthesis, 1973, p. 71.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.S. Matiichuk, M.A. Potopnyk, N.D. Obushak, 2009, published in Zhurnal Organicheskoi Khimii, 2009, Vol. 45, No. 5, pp. 728–733.
Rights and permissions
About this article
Cite this article
Matiichuk, V.S., Potopnyk, M.A. & Obushak, N.D. Synthesis and reactions of 3-(3-ethoxycarbonyl-1-phenyl-1H-pyrazol-4-yl)propenic acid. Russ J Org Chem 45, 712–718 (2009). https://doi.org/10.1134/S107042800905011X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107042800905011X