Abstract
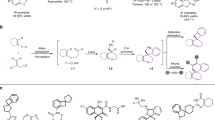
A general procedure has been developed for the introduction of hydrophobic alkyl groups into positions 6, 3, and 17 of steroid molecules via palladium-catalyzed Negishi reaction of halogen-substituted steroids with benzyl- and alkylzinc halides.
Similar content being viewed by others
References
Skoda-Foldes, R. and Kollar, L., Chem. Rev., 2003, vol. 103, p. 4095.
Potter, G.A., Barrie, S.E., Jarman, M., and Rowlands, M.G., J. Med. Chem., 1995, vol. 38, p. 2463.
Przezdziecka, A., Kurek-Tyrlik, A., and Wicha, J., Collect. Czech. Chem. Commun., 2002, vol. 67, p. 1658.
Arcadi, A., Burini, A., Cacchi, S., Delmastro, M., Marinelli, F., and Pietroni, B., Synlett, 1990, p. 47.
Ciattini, P.G., Morera, E., and Ortar, G., Tetrahedron Lett., 1990, vol. 31, p. 1889.
Felpin, F.-X., J. Org. Chem., 2005, vol. 70, p. 8575.
Chao, J., Ling, Y., Liu, X., Luo, X., and Brodie, A.M.H., Steroids, 2006, vol. 71, p. 585.
Cleve, A., Gunter, N., Ottow, E., Scholz, S., and Schwede, W., Tetrahedron, 1995, vol. 51, p. 5563.
Oh-e, T., Miyaura, N., and Suzuki, A., J. Org Chem., 1993, vol. 58, p. 2201.
Gravett, E.C., Hilton, J.P., Jones, K., and Romero, F., Tetrahedron Lett., 2001, vol. 42, p. 9081.
Ciattini, P.G., Morera, E., and Ortar, G., Tetrahedron Lett., 1992, vol. 33, p. 4815.
Skoda-Foldes, R., Pfeiffer, P., Kollar, L., Horvath, J., and Tuba, Z., Steroids, 2002, vol. 67, p. 709.
Tuozzi, A., Lo Sterzo, C., Sperandio, A., and Bocelli, G., Tetrahedron, 1999, vol. 55, p. 461.
Liu, Z. and Meinwald, J., J. Org. Chem., 1996, vol. 61, p. 6693.
Ciattini, P.G., Morera, E., and Ortar, G., Tetrahedron Lett., 1994, vol. 35, p. 2405.
Stang, P.J. and Treptow, W., Synthesis, 1980, p. 283.
Skoda-Foldes, R., Kollar, L., Horvath, J., and Tuba, Z., Steroids, 1995, vol. 60, p. 791.
Levina, I.S., Russ. Chem. Rev., 1998, vol. 67, p. 975.
Lukashev, N.V., Latyshev, G.V., Donez, P.A., Skryabin, G.A., and Beletskaya, I.P., Synthesis, 2005, p. 1578.
Lukashev, N.V., Latyshev, G.V., Donez, P.A., Skryabin, G.A., and Beletskaya, I.P., Synthesis, 2006, p. 533.
Donets, P.A., Latyshev, G.V., Lukashev, N.V., and Beletskaya, I.P., Izv. Ross. Akad. Nauk, Ser. Khim., 2007, p. 486.
Huo, S., Org. Lett., 2003, vol. 5, p. 423.
Latyshev, G.V., Lukashev, N.V., and Beletskaya, I.P., Russ. J. Org. Chem., 2007, vol. 43, p. 933.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Prof. M. Yus on his 60th anniversary
Original Russian Text © G.V. Latyshev, N.V. Lukashev, I.P. Beletskaya, 2008, published in Zhurnal Organicheskoi Khimii, 2008, Vol. 44, No. 6, pp. 797–801.
Rights and permissions
About this article
Cite this article
Latyshev, G.V., Lukashev, N.V. & Beletskaya, I.P. Pd-catalyzed alkylation of halogen-substituted steroids with organozinc compounds. Russ J Org Chem 44, 785–790 (2008). https://doi.org/10.1134/S107042800806002X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107042800806002X