Abstract
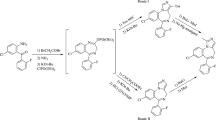
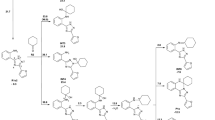
By a reaction of 2-methyl-4-chloroquinolines with o-mercaptoaniline under various conditions synteses were performed of substituted in the benzene ring 4-[(2-aminophenyl)thio]-2-methylquinolines and (4E)-4-[(2-mercaptophenyl)imino]-2-methyl-1,4-dihydroquinolines that were respectively by isomerization or rearrangement converted into 4-[(2-mercaptophenyl)amino]-2-methylquinolines.
Similar content being viewed by others
References
Okada, E. and Tsukushi, N., Heterocycles, 2000, vol. 53, p. 709.
Skrzypek, L. and Suwinska, K., Heterocycles, 2002, vol. 57, p. 2035.
Beilst., H, vol. 20, p. 392.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.A. Avetisyan, I.L. Aleksanyan, L.P. Ambartsumyan, 2008, published in Zhurnal Organicheskoi Khimii, 2008, Vol. 44, No. 5, pp. 732–735.
Rights and permissions
About this article
Cite this article
Avetisyan, A.A., Aleksanyan, I.L. & Ambartsumyan, L.P. Synthesis of substituted in benzene ring 4-[(2-aminophenyl)thio]-, 4-[(2-mercaptophenyl)amino]-2-methylquinolines and (4E)-4-[(2-mercaptophenyl)imino]-2-methyl-1,4-dihydroquinolines. Russ J Org Chem 44, 723–727 (2008). https://doi.org/10.1134/S1070428008050151
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428008050151