Abstract
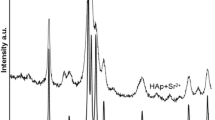
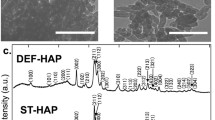
The physicochemical and sorption properties of hydroxyapatite synthesized in the presence of oxyethylidenediphosphonic acid, as crystallization inhibitor, were studied. The effect of acid concentration and calcium phosphate reagents was established on the sorption properties of hydroxyapatite with respect to ions Co2+, Pb2+, and Sr2+. It was shown that the sorption capacity of amorphous hydroxyapatite obtained in the presence of 1.0 mol % oxyethylidenediphosphonic acid, for Co2+ and Sr2+ ions, increases by 2–2.5 times, and for Pb2+ ions, by 4–5 times compared to crystalline hydroxyapatite obtained without acid additives. It was revealed that the observed differences in the sorption capacity of amorphous and crystalline hydroxyapatite with respect to the studied metal ions are due to different mechanisms of their adsorption.
Similar content being viewed by others
References
Sugiyama, S., Ichii, T., Fujisawa, M., Kawashiro, K., Tomida, T., Shigemoto, N., and Hayashi, H., J. Colloid Interface Sci., 2003, vol. 259, no. 2, pp. 408–410.
Shashkova, I.L., Rat'ko, A.I., and Kitikova, N.V., Colloid Surf. A, 1999, vol. 160, no. 3, pp. 207–215.
Shul'ga, N.V., Shashkova, I.L., and Samuskevich, V.V., Russ. J. Appl. Chem., 1999, vol. 72, no. 11, pp. 1963–1969.
Smiciklas, I., Onjia, A., Raicevic, S., Janackovic, D., and Mitric, M., J. Hazard. Mater., 2008, vol. 152, pp. 876–884.
Gomez del Rio, J.A., Morando, P.J., and Cicerone, D.S., J. Environ. Manage., 2004, vol. 71, pp. 169–177.
Wakamura, M., Kandori, K., and Ishikawa, T., Colloid Surf. A, 1998, vol. 142, pp. 107–116.
Tarasevich, Yu.I., Shkutkova, E.V., and Janusz, W., J. Water Chem. Technol., 2012, vol. 34, no. 3, pp. 125–132.
Lazic, S. and Vukovic, Z., J. Radioanal. Nucl. Chem., 1991, vol. 149, no. 1, pp. 161–168.
Stötzel, C., Müller, F. A., Reinert, F., Niederdraenk, F., Barralet, J.E., and Gbureck, U., Colloids Surf. B, 2009, vol. 74, pp. 91–95.
Lazarevic, S., Jankovic-Castvan, I., Tanaskovic, D., Pavicevic, V., Janackovic, Dj., and Petrovic, R., J. Environ. Eng., 2008, vol. 134, no. 8, pp. 683–688.
Elouear, Z., Bouzid, J., Boujelben, N., Feki, M., Jamoussi, F., and Montiel, A, J. Hazard. Mater., 2008, vol. 156, pp. 412–420.
Ivanets, A.I., Kitikova, N.V., Shashkova, I.L., Oleksiienko, O.V., Levchuk, I., and Sillanpää, M., J. Water Proc. Eng., 2016, vol. 9, pp. 246–253.
Oliva, J., Pablo, J.D., Cortina, J.-L., Cama, J., and Ayora, C., J. Hazard. Mater., 2011, vol. 194, pp. 312–323.
Smiciklas, I., Dimovic, S., Sljivic, M., and Plecas, I., J. Environ. Sci. Health. Part A, 2007, vol. 43, pp. 210–217.
Goto, T. and Sasaki, K., Ceram. Int., 2014, vol. 40, pp. 10777–10785.
Ivanets, A., Kitikova, N., Shashkova, I., Matrunchik, Y., and Kul'bitskaya, L., Environ. Technol. Innov., 2016, vol. 6, pp. 152–164.
Fernando, M.S., Silva, R.M., and Silva, K.M.N., Appl. Surf. Sci., 2015, vol. 351, pp. 95–103.
Posner, A.S. and Betts, F., Acc. Chem. Res., 1975, vol. 8, pp. 273–281.
Posner, A.S., Betts, F., and Blumenthal, N.C., Prog. Cryst. Growth Charact, 1980, vol. 3, pp. 49–64.
Boskey, A.L., J. Dent. Res., 1997, vol. 76, pp. 1433–1436.
Betts, F., Blumenthal, N. C., Posner, A.S., Becker, G.L., Lehninger, A.L., Proc. Nat. Acad. Sci. USA., 1975, vol. 72, pp. 2088–2090.
Onuma, K. and Ito, A., Chem. Mater., 1998, vol. 10, pp. 3346–3351.
Kanzaki, N., Onuma, K., Treboux, G., Tsutsumi, S., and Ito, A., J. Phys. Chem. B, 2000, vol. 104, pp. 4189–4194.
Ding, H., Pan, H., Xu, X., and Tang, R., Cryst. Growth Des., 2014, vol. 14, no. 2, pp. 763–769.
Nowak, B., Water Res., 2003, vol. 37, pp. 2533–2546.
Amjad, Z., Langmuir, 1987, vol. 3, pp. 1063–1069.
Ding, G.-J., Zhu, Y.-J., Qi, C., Sun, T.-W., Wu, J., Chen, F., RSC Adv., 2015, vol. 5, pp. 40154–40162.
Feng, Y., Gong, J.-L., Zeng, G.-M., Niu, Q.-Y., Zhang, H.- Y., Niu, C.-G., Deng, J.-H., Yan, M., Chem. Eng. J., 2010, vol. 162, pp. 487–494.
Author information
Authors and Affiliations
Corresponding author
Additional information
Russian Text © The Author(s), 2019, published in Zhurnal Prikladnoi Khimii, 2019, Vol. 92, No. 5, pp. 590–598.
Rights and permissions
About this article
Cite this article
Shashkova, I.L., Ivanets, A.I. & Kitikova, N.V. Sorption of Co2+, Pb2+, and Sr2+ Ions on Hydroxyapatite, Synthesized in the Presence of Oxyethylidenediphosphonic Acid. Russ J Appl Chem 92, 625–633 (2019). https://doi.org/10.1134/S1070427219050070
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427219050070