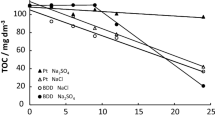
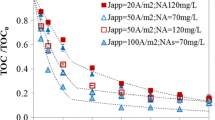
Abstract
Electrochemical oxidation for degradation of industrial dye Methyl Orange in aqueous sulfate solutions with various electrocatalytic materials: boron-doped diamond electrode and electrode based on titanium and ruthenium oxides. The influence exerted by the main working parameters of electrolysis (current density, concentration of Methyl Orange, pH) on the discoloration efficiency and on the chemical oxygen demand (COD) was examined. It was shown that an increase in the current density and a decrease in the pollutant concentration improve the process efficiency. However, this leads to an increase in the specific electric energy consumption per unit mass of COD. It was found that the boron-doped diamond electrode is a more efficient electrocatalytic material, compared with electrode based on titanium and ruthenium oxides. At low concentrations of Methyl Orange (<50 mg L–1), there exists the possibility in principle of using the electrode based on titanium and ruthenium oxides not only for discoloration, but also for making lower the COD level.
Similar content being viewed by others
References
Kant, R., Nat. Sci. 2012, vol. 4, pp. 22–26.
Przystaś, W., Zabłocka-Godlewska, E., and Grabińska-Sota, E., Water Air Soil Pollut., 2012, vol. 223, pp. 1581–1592.
Holkar, C.R., Jadhav, A.J., Pinjari, D.V., Mahamuni, N.M., and Pandit, A.B., J. Environ. Manag., 2016, vol. 182, pp. 351–366.
Robinson, T., McMullan, G., Marchant, R., and Nigam, P., Bioresour. Technol., 2001, vol. 77, pp. 247–255.
Kharlamova, T.A. and Aliev, Z.M., Russ. J. Electrochem., 2016, vol. 52, pp. 251–259.
Brillas, E. and Martínez-Huitle, C.A., Appl. Catal., B, 2015, vols. 166–167, pp. 603–643.
Martínez-Huitle, C.A. and Brillas, E., Appl. Catal., B, 2009, vol. 87, pp. 105–145.
Oturan, M.A., J. Appl. Electrochem., 2000, vol. 30, pp. 475–482.
Martínez-Huitle, C.A. and Ferro, S., Chem. Soc. Rev., 2006, vol. 35, pp. 1324–1340.
Comninellis, C., Electrochim. Acta, 1994, vol. 39, pp. 1857–1863.
Martínez-Huitle, C.A., Dos Santos, E.V., De Araújo, D.M., and Panizza, M., J. Electroanal. Chem., 2012, vol. 674, pp. 103–107.
Enache, T.A., Chiorcea-Paquim, A., Fatibello-Filho, O., and Oliveira-Brett, A.M., Electrochem. Commun., 2009, vol. 11, pp. 1342–1345.
Marselli, B., Garcia-Gomez, J., Michaud, P.A., Rodrigo, M.A., and Comninellis, C., J. Electrochem. Soc., 2003, vol. 150, pp. 79–83.
Panizza, M. and Cerisola, G., Electrochim. Acta, 2005, vol. 51, pp. 191–199.
Rodrigo, M.A., Cañizares, P., Sánchez-Carretero, A., and Sáez, C., Catal. Today, 2010, vol. 151, pp. 173–177.
Deborde, M. and Von-Gunten, U., Water Res., 2008, vol. 42, pp. 13–51.
Morais, C.C.O, Da Silva, A.J.C., Ferreira, M.B., De Araújo, D.M., Zanta, C.L.P.S., and Castro, S.S.L., Electrocatal., 2013, vol. 4, pp. 312–319.
Fisher, H., Praktikum po obshchei khimii: Vvodnyi kurs po ekologicheski bezopasnoi programme s eksperimentami po regeneratsii khimicheskikh reaktivov, Ch. 2, Organicheskaya i fizicheskaya khimiya (Practical Course of General Chemistry, Introductory Course of Ecologically Safe Program with Experiments on Regeneration of Chemical Reagents, Part 2, Organic and Physical Chemistry), Novosibirsk: Nauka, 2002. 2nd ed.
Fedorova, A.I. and Nikol’skaya, A.N., Praktikum po ekologii i okhrane okruzhayushchei sredy (Practical Course of Ecology and Environment Protection), Moscow: VLADOS, 2003.
Morsi, M.S., Al-Sarawy, A.A., and Shehab El-Dein, W.A., Desalin. Water Treat., 2011, vol. 26, pp. 301–308.
Hamza, M., Abdelhedi, R., Brillas, E., and Sires, I., J. Electroanal. Chem., 2009, vol. 627, pp. 41–50.
Panizza, M. and Cerisola, G., Ind. Eng. Chem. Res., 2008, vol. 47, pp. 6816–6820.
Matzek, L.W. and Carter, K.E., Chemosphere, 2016, vol. 151, pp. 178–188.
Thiam, A., Sirés, I., Garrido, J.A., Rodríguez, R.M., and Brillas, E., Sep. Purif. Technol., 2015, vol. 140, pp. 43–52.
Acero, J.L., Benítez, F.J., Real, F.J., and Rodríguez, E., Sep. Purif. Technol., 2018, vol. 201, pp. 41–50.
Song, S., Fan, J., He, Z., Zhan, L., Liu, Z., Chen, J., and Xu, X., Electrochim. Acta, 2010, vol. 55, pp. 3606–3613.
Carneiro, P.A., Fugivara, C.S., Nogueira, R.F.P., Boralle, N., and Zanoni, M.V.B., Port. Electrochim. Acta, 2003, vol. 21, pp. 49–67.
Hmani, E., Samet, Y., and Abdelhédi, R., Diamond Relat. Mater., 2012, vol. 30, pp. 1–8.
Moreno-Casillas, H.A., Cocke, D.L., Gomes, J.A.G., Morkovsky, P., Parga, J.R., and Peterson, E., Sep. Purif. Technol., 2007, vol. 56, pp. 204–211.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © T.A. Kenova, G.V. Kornienko, O.A. Golubtsova, V.L. Kornienko, N.G. Maksimov, 2018, published in Zhurnal Prikladnoi Khimii, 2018, Vol. 91, No. 9, pp. 1241–1251.
Rights and permissions
About this article
Cite this article
Kenova, T.A., Kornienko, G.V., Golubtsova, O.A. et al. Electrocatalytic Oxidation of Organic Pollutants on Boron-Doped Diamond and Ti–Ru Oxide Anodes in Sulfate Medium. Russ J Appl Chem 91, 1412–1421 (2018). https://doi.org/10.1134/S1070427218090021
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427218090021