Abstract
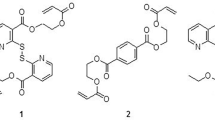
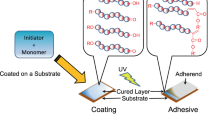
Fundamental study of how radicals are generated, with involvement of trialkylboranes combined with oxygen and organoelement peroxides, was carried out. It was shown that the complex process of gluing of polymeric materials with low-energy surface predominantly occurs under the action of trialkylborane unblocked from the amine complex and oxygen dissolved in the gluing formulation and also under the action of growth radicals produced in the trialkylborane–peroxide–acrylate system in the closed-system mode at room temperature. The curing process of the gluing formulation occurs in the course of time both in the bulk and on the substrate surface.
Similar content being viewed by others
References
Sonneshein, M.F., Webb, S.P., Kastl, P.E., et al., Macromolecules, 2004, vol. 37, pp. 7974–7978.
Okamura, H., Sudo, A., and Endo, T., Polymer Chem., Part A, 2009, vol. 47, pp. 6163–6167.
Zi, M., Zheng, Z., Liu, S., et al., Int. J. Adhes. Adhes., 2011, vol. 31, pp. 36–42.
Sonnenshein, M.F., Webb, S.P., Redwine, O.D., et al., Macromolecules, 2006, vol. 39, pp. 2507–2513.
Kablov, E.N. and Reznichenko, S.V., Kleyashchie materialy (Adhesive Materials), Moscow: KIR, 2002.
Trizno, M.S. and Moskalev, E.V., Klei i skleivanie (Adhesives and Gluing), Leningrad: Khimiya, 1980.
Kolesnikov, G.S. and Fedorova, L.S., Izv. Akad. Nauk SSSR, Otd. Khim. Nauk, 1957, no. 2, pp. 236–237.
Furukawa, J., Tsuruta, T., Imada, T., and Furutani, H., Macromol. Chem., 1959, vol. 31, no. 2, pp. 122–139.
Davies, A.G., Ingold, K.U., Roberts, B.R., and Tudor, R., J. Chem. Soc. B, 1971, pp. 698–712.
Zaremski, M.Y., Budanov, D.V., Romanov, S.A., et al., Polym. Sci. Ser. B, 2011, vol. 53, nos. 1–2, pp. 1–9.
Zaremski, M.Yu., Garina, E.S., Gurskii, M.E., and Bubnov, Yu.N., Polym. Sci. Ser. B, 2013, vol. 55, nos. 5–6, pp. 304–326.
Zhang Z.C., Chung T.C.M., Macromolecules, 2006, vol. 39, pp. 5184–5189.
Razuvaev, G.A., Dodonov, V.A., Tsvetkov, V.G., et al., Vysokomol. Soedin., 1988, vol. 30, no. 2, pp. 146–150.
Dodonov, V.A., Morozov, O.S., Grishin, D.F., et al., Dokl. Akad. Nauk SSSR, 1980, vol. 255, no. 5, pp. 1123–1127.
Dodonov, V.A., Lomakin, S.S., and Gulenova, M.V., Klei, Germetiki, Tekhnol., 2013, no. 11, pp. 29–33.
Dodonov, V.A., Starostina, T.I., Kuropatov, V.A., et al., Russ. J. Appl. Chem., 2017, vol. 90, no. 1, pp. 77–83.
Dodonov, V.A., Grishin, D.F., and Aksenova, I.N., Vysokomol. Soedin., Ser. B, 1993, vol. 35, no. 12, pp. 2070–2072.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.A. Dodonov, T.I. Starostina, 2017, published in Zhurnal Prikladnoi Khimii, 2017, Vol. 90, No. 10, pp. 1410−1415.
Rights and permissions
About this article
Cite this article
Dodonov, V.A., Starostina, T.I. Specific Features of Radical Formation in the Trialkylborane–Oxygen System and the Gluing Mechanism of Acrylate Composites. Russ J Appl Chem 90, 1722–1726 (2017). https://doi.org/10.1134/S107042721710024X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107042721710024X