Abstract
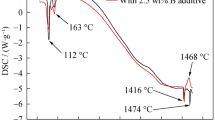
Reduction of zirconium dioxide with boron carbide and nanofibrous carbon in argon yielded a highly dispersed powder of zirconium diboride. Characteristics of zirconium diboride powders were examined by various analytical methods. The material obtained is represented by a single phase, zirconium diboride. Powder particles are for the most part aggregated. The average size of particles and aggregates is 10.9–12.9 μm with a wide size distribution. The specific surface area of the samples is 1.8–3.6 m2 g–1. The oxidation of zirconium diboride begins at a temperature of 640°C The optimal synthesis parameters were determined: ZrO2: B4C: C molar ratio of 2: 1: 3 (in accordance with stoichiometry), process temperature 1600–1700°C, synthesis duration 20 min.
Similar content being viewed by others
References
Serebryakova, T.I., Neronov, V.A., and Peshev, P.D., Vysokotemperaturnye boridy (High-Temperature Borides), Moscow: Metallurgiya, Chelyabinsk. otd., 1991.
Mroz, C., Am. Ceram. Soc. Bull., 1995, vol. 74, pp. 158–159.
Fahrenholtz, G., Hilmas, G.E., Talmy, I.G., and Zaykoski, J.A., J. Am. Ceram. Soc., 2007, vol. 90, pp. 1347–1364.
Sonber, J.K. and Suri, A.K., Adv. Appl. Ceram., 2011, vol. 110, pp. 321–334.
Monteverde, F., Savino, R., and Fumo, M.S.D., Corros. Sci., 2011, vol. 53, pp. 922–929.
Zou, X., Fu, Q., Liu, L., et al., Surf. Coat. Technol., 2013, vol. 226, pp. 17–21.
Neuman, E.W., Hilmas, G.E., and Fahrenholtz, W.G., J. Eur. Ceram. Soc., 2013, vol. 33, pp. 2889–2899.
Mishra, S.K., Das, S., and Ramchandrarao, P., J. Mater. Res., 2002, vol. 17, pp. 2809–2814.
Setoudeh, N. and Welham, N.J., J. Alloys Compd., 2006, vol. 420, pp. 225–228.
Khanra, A.K., Mater. Res. Bull., 2007, vol. 42, pp. 2224–2229.
Khanra, A.K., Pathak, L.C., and Godkhindi, M.M., J. Mater. Process. Technol., 2008, vol. 202, pp. 386–390.
Jalaly, M., Bafghi, M.Sh., Tamizifar, M., and Gotor, E.J., J. Alloys Compd., 2014, vol. 588, pp. 36–41.
Zhou, F., Zhengyi, F., Hao, W., et al., J. Wuhan Univ. Technol.- Mater. Sci. Ed., 2005, vol. 20, pp. 87–89.
Rao, L., Gillan, E.G., and Caner, R.B., J. Mater. Res., 1995, vol. 10, pp. 353–361.
Millet, P. and Hwang, T., J. Mater. Sci., 1996, vol. 31, pp. 351–355.
Ran, S., Van der Biest, O., and Vleugels, J., J. Am. Ceram. Soc., 2010, vol. 93, pp. 1586–1590.
Li, R., Lou, H., Yin, S., et al., J. Alloys Compd., 2011, vol. 509, pp. 8581–8583.
Guo, W.-M. and Zhang, G.-J., J. Am. Ceram. Soc., 2011, vol. 94, pp. 3702–3705.
Chen, B.L. Yang, L., Heng, H., et al., J. Solid State Chem., 2012, vol. 194, pp. 219–224.
Radev, D.D. and Klissurski, D., J. Mater. Synth. Process, 2001, vol. 9, pp. 131–136.
Çamurlu, H.E. and Maglia, F., J. Eur. Ceram. Soc., 2009, vol. 29, pp. 1501–1506.
Guo, S., Hu, C., and Kagawa, Y., J. Am. Ceram. Soc., 2011, vol. 94, pp. 3643–3647.
Lou, H., Li, R., Zhang, Y., et al., Rare Met., 2011, vol. 30, pp. 548–551.
Jung, E.-Y., Kim, J.-H., Jung, S.-H., and Choi, S.-C., J. Alloys Compd., 2012, vol. 538, pp. 164–168.
Schwab, S.T., Stewart, S.A., Dudeck, K.W., et al., J. Mater. Sci., 2004, vol. 39, pp. 6051–6055.
Yan, Y., Huang, Z., Dong, S., and Jiang, D., J. Am. Ceram. Soc., 2006, vol. 89, pp. 3585–3588.
Li, R., Zhang, Y., Lou, H., et al., J. Sol-Gel Sci. Technol., 2011, vol. 58, pp. 580–585.
Zhang, Y., Li, R., Jiang, Y., et al., J. Solid State Chem., 2011, vol. 184, pp. 2047–2052.
Hie, C., Chen, M., Wei, X., et al., J. Am. Ceram. Soc., 2012, vol. 95, pp. 866–869.
Cheng, G., Int. J. Refract. Met. Hard Mater.. 2013, vol. 36, pp. 149–153.
Guo, W.-M. and Zhang, G.-J., J. Am. Ceram. Soc., 2009, vol. 92, pp. 264–267.
Sonber, J.K., Murthy, T.S.R.Ch., Subramanian, C., et al., Int. J. Refract. Met. Hard Mater., 2011, vol. 29, pp. 21–30.
Qiu, H.-Y., Guo, W.-M., Zou, J., and Zhang, G.-J., Powder Technol., 2012, vol. 217, pp. 462–466.
Kuvshinov, G.G., Popov, M.V., Tonkodubov, S.E., et al., Russ. J. Appl. Chem., 2016, vol. 89, no. 11, pp. 1777–1785.
Krutskii, Yu.L, Bannov, A.G., Sokolov, V.V., et al., Nanotech. Russ., 2013, no. 8, pp. 191–198.
Krutskii, Yu.L., Dyukova, K.D., Bannov, A.G., et al., Russ. J. Adv. Mater., 2015, no. 3, pp. 55–62).
Krutskii, Yu.L., Bannov, A.G., Antonova, E.V., et al., Ceram. Interfaces, 2016, vol. 43, pp. 3212–3217.
Svoistva, poluchenie i primenenie tugoplavkikh soedinenii: Spravochnik (Properties, Synthesis, and Application of High-Melting Compound: Refernce Book), Kosolapova, T.Ya., Ed., Moscow: Metallurgiya, 1986.
Kornienko, E.E., Nikulina, A.A., Bannov, A.G., et al., Metal Work. & Mat. Sci., 2016, no. 4, pp. 52–56.
Novyi spravochnik khimika i tekhnologa. Osnovnye svoistva neorganicheskikh, organicheskikh i elementoorganicheskikh soedinenii: Spravochnik (New Chemist’s and Technologist’s Handbook, Basic Properties of Organoelement Compounds: Reference Book), Skvortsov, N.K., Moskvin, A.V., Simanova, S.A., and Stolyarova, V.A., Eds., St. Petersburg: ANO NIO Mir i sem’ya, 2002.
Svoistva elementov: Spravochnik (Properties of Elements: Reference Book), Drits, M.E., Ed., Moscow: Metallurgiya, 1985.
West, A.R., Solid State Chemistry and Its Applications, Chichester: Wiley, 1984.
Blott, S.J. and Pye, K., Earth Surf. Processes Landforms, 2001, vol. 26, pp. 1237–1248.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © Yu.L. Krutskii, E.A. Maksimovskii, M.V. Popov, O.V. Netskina, T.M. Krutskaya, N.Yu. Cherkasova, T.S. Kvashina, E.A. Drobyaz, 2017, published in Zhurnal Prikladnoi Khimii, 2017, Vol. 90, No. 10, pp. 1295−1302.
Rights and permissions
About this article
Cite this article
Krutskii, Y.L., Maksimovskii, E.A., Popov, M.V. et al. Synthesis of Highly Dispersed Zirconium Diboride for Fabrication of Special-Purpose Ceramic. Russ J Appl Chem 90, 1579–1585 (2017). https://doi.org/10.1134/S1070427217100044
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427217100044