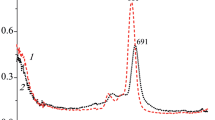
Abstract
Tetrakis{5,6-bis(4-tert-butylphenyl)pyrazino[2,3-c]}porphyrazine and tetra(4-tert-butyl)phthalocyanine have been synthesized, and their acid–base properties and electronic absorption and fluorescence spectra in acetonitrile and toluene at 298 and 295 K, respectively, have been studied. The synthesized compounds have been identified by electronic absorption and1H NMR spectroscopy and mass spectrometry. The effect of substituents in the molecular fragments of the macrocycle on the electronic and optical properties of the synthesized compounds has been estimated.






Similar content being viewed by others
REFERENCES
Berezin, B.D., Koordinatsionnye soedineniya porfirinov i ftalotsianina (Coordination Compounds of Porphyrins and Phthalocyanine), Moscow: Nauka, 1978.
Stepanov, B.I., Vvedenie v khimiyu i tekhnologiyu organicheskikh krasitelei (Introduction to the Chemistry and Technology of Organic Dyes), Moscow: Khimiya, 1984.
Porfiriny: spektroskopiya, elektrokhimiya, primenenie (Porphyrins: Spectroscopy, Electrochemistry, and Application), Enikolopyan, N.S., Ed., Moscow: Nauka, 1987.
Stillman, M.J. and Nyokong, T., Phthalocyanines: Properties and Applications, Leznoff, C.C. and Lever, A.B.P., Eds., New York: VCH, 1989, vol. 1, p. 133.
Liu, Z., Zhang, X., Zhang, Y., and Jiang, J., Spectrochim. Acta, Part A, 2007, vol. 67, no. 5, p. 1232. https://doi.org/10.1016/j.saa.2006.10.013
Nonlinear Optics of Organic Molecules and Polymers, Nalwa, H.S. and Miyata, S., Eds., Boca Raton: CRC, 1997.
Sheinin, V.B. and Ivanova, Yu.B., Russ. J. Phys. Chem. A, 2007, vol. 81, no. 8, p. 1250. https://doi.org/10.1134/S0036024407080134
Petrov, O.A., Osipova, G.V., and Khelevina, O.G., Macroheterocycles, 2009, vol. 2, no. 2, p. 151.
Bershtein, I.Ya. and Kaminskii, Yu.L., Spektrofotometricheskii analiz v organicheskoi khimii (Spectrophotometric Analysis in Organic Chemistry), Leningrad: Khimiya, 1986.
Andrianov, V.G. and Malkova, O.V., Macroheterocycles, 2009, vol. 2, no. 2, p. 130.
Hirao, H., J. Phys. Chem. A, 2011, vol. 115, no. 33, p. 9308. https://doi.org/10.1021/jp2052807
Dhami, S., Mello, A.D., Rumbles, G., Bishop, S.M., Phillips, D., and Beeby, A., Photochem. Photobiol., 1995, vol. 61, no. 4, p. 341. https://doi.org/10.1111/j.1751-1097.1995.tb08619.x
Whalley, M., J. Chem. Soc., 1961, p. 866. https://doi.org/10.1039/JR9610000866
Lakowicz, J.R., Principles of Fluorescence Spectroscopy, New York: Springer, 2006, 3rd ed.
Freyer, W., Mueller, S., and Teuchner, K.J., J. Photochem. Photobiol., C, 2004, vol. 163, nos. 1–2, p. 231. https://doi.org/10.1016/j.jphotochem.2003.12.003
Vachova, L., Novakova, V., Kopecky, K., Miletin, M., and Zimcik, P., Dalton Trans., 2012, vol. 41, no. 38, p. 11651. https://doi.org/10.1039/c2dt31403g
Karyakin, Yu.V. and Angelov, I.I., Chistye khimicheskie reaktivy (Pure Chemicals), Moscow: Khimiya, 1974, 4th ed.
Ivanova, Yu.B., Churakhina, Yu.I., and Mamardashvili, N.Zh.,Russ. J. Gen. Chem., 2008, vol. 78, no. 4, p. 673. https://doi.org/10.1134/S1070363208100265
Ivanova, Yu.B., Sheinin, V.B., and Mamardashvili, N.Zh., Russ. J. Gen. Chem., 2007, vol. 77, no. 8, p. 1458. https://doi.org/10.1134/S1070363207080270
Ivanova, Yu.B. and Mamardashvili, N.Zh., J. Fluoresc., 2017, vol. 27, no. 5, p. 303. https://doi.org/10.1007/s10895-016-1958-1
Ivanova, Yu.B., Mamardashvili, N.Zh., Semeikin, A.S., and Glazunov, A.V., Russ. J. Org. Chem., 2010, vol. 46, no. 6, p. 917. https://doi.org/10.1134/S1070428010060230
Kruk, N.N., Starukhin, A.S., Mamardashvili, N.Zh., Sheinin, V.B., and Ivanova, Yu.B., RU Patent no. 2345352, 2009.
Ivanova, Yu.B., Chizhova, N.V., Pukhovskaya, S.G., and Mamardashvili, N.Zh., Russ. J. Gen. Chem., 2014, vol. 84, no. 5, p. 939. https://doi.org/10.1134/S1070363214050260
Nguyen, N.T., Mamardashvili, G.M., Kulikova, O.M., Scheblykin, I.G., Mamardashvili, N.Z., and Dehaen, W., RSC Adv., 2014, vol. 4, no. 38, p. 19703. https://doi.org/10.1039/C3RA45660A
Funding
This study was performed under financial support by the Russian Science Foundation (project no. 19-73-20079) using the facilities of the Upper Volga Regional Center for Physicochemical Studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare the absence of conflict of interest.
Rights and permissions
About this article
Cite this article
Ivanova, Y.B., Dmitrieva, O.A., Khrushkova, Y.V. et al. Synthesis and Acid–Base, Absorption, and Fluorescence Properties of Phthalocyanine Derivatives. Russ J Gen Chem 90, 852–857 (2020). https://doi.org/10.1134/S1070363220050151
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363220050151