Abstract
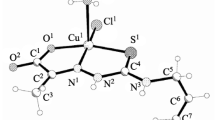
N-(Prop-2-en-1-yl)hydrazonocarbothioamide reacts with iodoethane in methanol with further addition of 2-hydroxybenzaldehyde to form hydroiodide of carbamohydrazonothioate (HL·HI). The coordination compounds were obtained by interaction of HL or HL·HI with copper, nickel, cobalt and iron salts CuLХ·nH2O [X = Cl–, Br–, NO3–; n = 0–1], Ni(L)2·HI·CH3OH, Сo(L)2X [X = I–, NO3–] and Fe(L)2NO3. The structures of three complexes were established by single crystal X-ray analysis. The synthesized complexes exhibit selective antimicrobial and antifungal activity against a series of standard microorganisms and fungi in the concentration range of 30–500 μg/mL. In addition, nickel and iron complexes selectively inhibit the growth and proliferation of cancer cells and do not adversely affect normal cells.








Similar content being viewed by others
REFERENCES
Beraldo, H. and Gambino, D., Mini Rev. Med. Chem., 2004, vol. 4, no. 1, p. 31. https://doi.org/10.2174/1389557043487484
Saryan, L.A., Ankel, E., Krishnamurti, C., Petering, D.H., and Elford, H., J. Med. Chem., 1979, vol. 22, no. 10, p. 1218. https://doi.org/10.1021/jm00196a013
Pahontu, E., Fala, V., Gulea, A., Poirier, D., Tapcov, V., and Rosu, T., Molecules, 2013, vol. 18, no. 8, p. 8812. https://doi.org/10.3390/molecules18088812
Turk, S.R., Shipman, C., and Drach, J.C., J. Gen. Virology, 1986, vol. 67, no. 8, p. 1625. https://doi.org/10.1099/0022-1317-67-8-1625
Yamazaki, C., Canad. J. Chem., 1975, vol. 53, no. 4, p. 610. https://doi.org/10.1139/v75-085
Botoshanskii, M., Bourosh, P.N., Revenko, M.D., Korzha, I.D., Simonov, Y.A., and Panfilie, T., J. Struct. Chem., 2009, vol. 50, no. 1, p. 181. https://doi.org/10.1007/s10947-009-0026-y
Leovac, V.M., Češljević, V.I., Vojinović-Ješić, L.S., Divjaković, V., Jovanović, L.S., Szécsényi, K.M., and Rodić, M.V., Polyhedron, 2009, vol. 28, no. 16, p. 3570. https://doi.org/10.1016/j.poly.2009.07.045
Rodić, M.V., Leovac, V.M., Jovanović, L.S., Vojinović Ješić, L.S., Divjaković, V., and Češljević, V.I., Polyhedron, 2012, vol. 46, no. 1, p. 124. https://doi.org/10.1016/j.poly.2012.08.011
Petrovic, D.M., Petrovic, A.F., Leovac, V.M., and Lukic, S.R., J. Thermal Anal., 1994, vol. 41, no. 5, p. 1165. https://doi.org/10.1007/bf02547205
Malik, M. and Phillips, D., Austral. J. Chem., 1974, vol. 27, no. 5, p. 1133. https://doi.org/10.1071/ch9741133
Takjoo, R., Mague, J.T., Akbari, A., and Ahmadi, M., J. Coord. Chem., 2013, vol. 66, no. 22, p. 3915. https://doi.org/10.1080/00958972.2013.856420
Pahontu, E., Usataia, I., Graur, V., Chumakov, Yu., Petrenko, P., Gudumac, V., and Gulea, A., Appl. Organometal. Chem., 2018, vol. 32, no. 12, p. 4544. https://doi.org/10.1002/aoc.4544
Türkkan, B., Sarıboğa, B., and Sarıboğa, N., Transition Metal Chem., 2011, vol. 36, no. 6, p. 679. https://doi.org/10.1007/s11243-011-9518-7
Şahin, M., Bal-Demirci, T., Pozan-Soylu, G., and Ülküseven, B., Inorg. Chim. Acta, 2009, vol. 362, no. 7, p. 2407. https://doi.org/10.1016/j.ica.2008.10.036
CrysAlisPro, Version 1.171.33.52 (release 06-11-2009 CrysAlis171.NET). Oxford Diffraction Ltd.
Sheldrick, G.M., Acta Crystallogr. (A), 2007, vol. 64, no. 1, p. 112. https://doi.org/10.1107/s0108767307043930
Spek, A.L., J. Appl. Crystallogr., 2003, vol. 36, no. 1, p. 7. https://doi.org/10.1107/s0021889802022112
Macrae, C.F., Edgington, P.R., McCabe, P., Pidcock, E., Shields, G.P., Taylor, R., and Van De Streek, J., J. Appl. Crystallogr., vol. 39, no. 3, p. 453. https://doi.org/10.1107/s002188980600731x
Gulea, A., Poirier, D., Roy, J., Stavila, V., Bulimestru, I., Tapcov, V., and Popovschi, L., J. Enzyme Inhibition Med. Chem., 2008, vol. 23, no. 6, p. 806. https://doi.org/10.1080/14756360701743002
ACKNOWLEDGMENTS
The authors express gratitude to O.S. Garbuz for the help in performance of biological tests of synthesized substances.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Gulea, А.P., Usataia, I.S., Graur, V.О. et al. Synthesis, Structure and Biological Activity of Coordination Compounds of Copper, Nickel, Cobalt, and Iron with Ethyl N'-(2-Hydroxybenzylidene)-N-prop-2-en-1-ylcarbamohydrazonothioate. Russ J Gen Chem 90, 630–639 (2020). https://doi.org/10.1134/S107036322004012X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107036322004012X