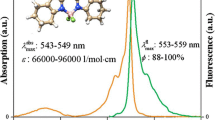
Abstract
4-[4-(2-Bromoacetyl)phenyl]-3-hydroxy-2H-chromen-2-one reacted with pyridine and 4-methylpyridine to give the corresponding quaternary pyridinium salts. The condensation of 1-{2-[4-(3-hydroxy-2-oxo-2H-chromen-4-yl)phenyl]-2-oxoethyl}-4-methylpyridinium bromide with 4-(dimethylamino)benzaldehyde afforded a new biscyanine dye whose electronic spectrum showed two absorption maxima originating from interactions of chromophores. The nature of electronic transitions in the dye molecule was analyzed by quantum chemical calculations.









Similar content being viewed by others
REFERENCES
Panigrahi, M., Dash, S., Patel, S., and Mishra, B.K., Tetrahedron, 2012, vol. 68, no. 3, p. 781. https://doi.org/10.1016/j.tet.2011.10.069
Shindy, H.A., Dyes Pigm., 2017, vol. 145, p. 505. https://doi.org/10.1016/j.dyepig.2017.06.029
Shindy, H.A., Mini-Rev. Org. Chem., 2012, vol. 9, no. 2, p. 209. https://doi.org/10.2174/157019312800604652
Kiprianov, A.I., Tsvet i stroenie tsianinovykh krasitelei (Color and Structure of Cyanine Dyes), Kiev: Naukova Dumka, 1979, p. 303.
Kachkovskii, A.D., Stroenie i tsvet polimetinovykh krasitelei (Structure and Color of Cyanine Dyes), Kiev: Naukova Dumka, 1989.
Mushkalo, I.L. and Shedov, I.F., Chem. Heterocycl. Compd., 1974, vol. 10, no. 11, p. 1309. https://doi.org/10.1007/BF01175085
Kiprianov, A.I., Russ. Chem. Rev., 1971, vol. 40, no. 7, p. 594. https://doi.org/10.1070/RC1971v040n07ABEH001942
Chernyuk, I.N., Yagodinets, P.I., and Shevchuk, M.I., Zh. Obshch. Khim., 1982, vol. 52, no. 3, p. 716.
Yagodinets, P.I., Russ. J. Gen. Chem., 1998, vol. 68, no. 8, p. 1256.
Kiprianov, A.I. and Mushkalo, I.L., Zh. Org. Khim., 1965, vol. 1, no. 4, p. 744.
Kiprianov, A.I. and Dyadyusha, G.G., Ukr. Khim. Zh., 1969, vol. 35, no. 6, p. 608.
Yelenich, O.V., Skrypska, O.V., Lytvyn, R.Z., Neshchadin, A.O., Obushak, M.D., Kachkovskii, A.D., and Yagodinets, P.I., Russ. J. Gen. Chem., 2014, vol. 84, no. 11, p. 2114. https://doi.org/10.1134/S1070363214110127
Yelenich, O.V., Lytvyn, R.Z., Skrypska, O.V., Pitkovych, Kh.Ye., Kachkovskii, A.D., Obushak, M.D., and Yagodinets, P.I., Russ. J. Gen. Chem., 2016, vol. 86, no. 8, p. 1838. https://doi.org/10.1134/S1070363214110127
Chernyuk, I.N., Yagodinets, P.I., and Pridan, V.E., Russ. J. Gen. Chem., 1997, vol. 67, no. 3, p. 427.
Yagodinets, P.I., Russ. J. Org. Chem., 1998, vol. 34, no. 1, p. 137.
Yagodinets, P.I., Russ. J. Gen. Chem., 1997, vol. 67, no. 9, p. 1482.
Yagodinets, P.I., Russ. J. Gen. Chem., 1998, vol. 68, no. 8, p. 1252.
Yagodinets’, P.І., Kachkovs’kii, O.D., and Skrips’ka, O.V., Zh. Org. Farm. Khіm., 2005, vol. 3, no. 2, p. 55.
Pridan, V.E., Chernyuk, I.N., Rogovik, L.I., and Bukachuk, O.M., Zh. Org. Khim., 1980, vol. 16, no. 9, p. 1973.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery, J.A.Jr., Vreven, T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C., and Pople, J.A., Gaussian 03, Revision B.03, Wallingford CT: Gaussian, 2003.
Hedvig, P., Experimental Quantum Chemistry, Budapest: Akadémiai Kiadó, 1975.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Skrypska, O.V., Lytvyn, R.Z., Rusnak, O.V. et al. Synthesis and Electronic Transitions of the Dye Based on 1-{2-[4-(3-Hydroxy-2-oxo-2H-chromen-4-yl)phenyl]-2-oxoethyl}-4-methylpyridinium Bromide. Russ J Gen Chem 90, 602–609 (2020). https://doi.org/10.1134/S1070363220040076
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363220040076