Abstract
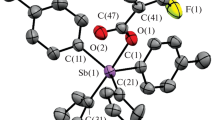
Binuclear antimony compounds with a bridging oxygen atom [Ph3SbOC(O)R]2O (R = CF2CF3, CF2CF2CF3), [(4-МеC6H4)3SbOC(O)CF2CF3]2O, [(3-FC6H4)3SbOC(O)R]2O (R = C6F5, CF2CF3) have been synthesized by reacting equimolar amounts of triarylantimony, carboxylic acid and tert-butyl hydroperoxide in diethyl ether. According to X-ray diffraction data, in the molecules of compounds obtained, the Sb atoms have a trigonal bipyramid coordination with carboxylate ligands and a bridging oxygen atom in axial positions. The intramolecular distances Sb···O with a carbonyl oxygen atom are less than the sum of the van der Waals radii of Sb and O by ~0.2–0.4 Å.
Similar content being viewed by others
References
Ferguson, G., Kaither, B., Glidewell, С., Ferguson, G., Kaither, B., Glidewell, C., and Smith, S.J., J. Organomet. Chem., 1991, vol. 419, p. 283. doi https://doi.org/10.1016/0022-328X(91)80241-B
Qin, W., Yasuike, S., Kakusawa, N., Qin, W., Yasuike, S., Kakusawa, N., Sugawara, Y., Kawahata, M., Yamaguchi, K., and Kurita, J., J. Organomet. Chem., 2008, vol. 693, p. 109. doi https://doi.org/10.1016/j.jorganchem.2007.10.030
Sharutin, V.V. and Sharutina, O.K., Russ. Chem. Bull., 2017, vol. 66, p. 707. doi https://doi.org/10.1007/s11172-017-1796-6
Sharutin, V.V., Sharutina, O.K., Reshetnikova, R.V., Lobanova, E.V., and Efremov, A.N., Russ. J. Inorg. Chem., 2017, vol. 62, p. 1450. doi https://doi.org/10.1134/S003602361711016X
Sharutin, V.V., Sharutina, O.K., and Efremov, A.N., Russ. J. Inorg. Chem., 2016, vol. 61, p. 43. doi https://doi.org/10.1134/S003602361601023X
Yu, L., Ma, Y-Q., Wang, G-C., and Li, J-S., Heteroatom. Chem., 2004, vol. 15, p. 32. doi https://doi.org/10.1002/hc.10208
Yu, L., Ma, Y-Q., Liu, R-C., Yu, L., Ma, Y.Q., Liu, R.C., Wang, G.C., Li, J.S., Du, G.H., and Hu, J.J., Polyhedron, 2004, vol. 23, p. 823. doi https://doi.org/10.1016/j.poly.2003.12.002
Hadjikakou, S.K., Ozturk, I.I., Banti, C.N., and Kourkoumelis, N., Hadjiliadis, N.J., Inorg. Biochem., 2015, vol. 153, p. 293. doi https://doi.org/10.1016/j.jinorgbio.2015.06.006
Ali, M.I., Rauf, M.K., Badshah, A., Ali, M.I., Rauf, M.K., Badshah, A., Kumar, I., Forsyth, C.M., Junk, P.C., Kedzierski, L., and Andrews, P.C., Dalton Trans., 2013, vol. 42, p. 16733. doi https://doi.org/10.1039/C3DT51382C
Sharutin, V.V., Sharutina, O.K., and Senchurin, V.S., Russ. J. Coord. Chem., 2014, vol. 40, p. 109. doi https://doi.org/10.1134/S1070328414020109
Quan, L., Yin, H., and Wang, D., Acta Crystallogr. (E), 2008, vol. 64, p. m349. doi https://doi.org/10.1107/S1600536808000676
Quan, L., Yin, H., and Wang, D., Acta Crystallogr. (E), 2009, vol. 65, p. m99. doi https://doi.org/10.1107/S1600536808042335
Gibbons, M.N. and Sowerby, D.B., J. Organomet. Chem., 1998, vol. 555, p. 271. doi https://doi.org/10.1016/S0022-328X(97)00759-6.
Sharutin, V.V., Pakusina, A.P., Nasonova, N.V., Sharutina, O.K., Gerasimenko, A.V., ansd Pushilin, M.A., Khimiya i Komp’yuternoe Modelirovanie. Butrlovsk. Soobshch. (Chemistry and Computer Simulation. Butlerov Reports), 2002, no. 11, p. 13.
Bruker (1998). SMART and SAINT-Plus. Versions 5.0. Data Collection and Processing Software for the SMART System. Bruker AXS Inc., Madison, Wisconsin, USA.
Bruker (1998). SHELXTL/PC. Versions 5.10. An Integrated System for Solving, Refining and Displaying Crystal Structures From Diffraction Data. Bruker AXS Inc., Madison, Wisconsin, USA.
Dolomanov, O.V., Bourhis, L.J., Gildea, R.J., Howard, J.A.K., and Puschmann, H.J., J. Appl. Cryst., 2009, vol. 42, p. 339. doi https://doi.org/10.1107/S0021889808042726
Sharutin, V.V. and Senchurin, V.S., Imennye reaktsii v khimii elementorganicheskikh soedinenii (Nominal Reaction in Chemistry of Organoelemental Compounds), Celyabinsk: Izd. Tsentr YuUrGU, 2011.
Glidewell, C., J. Orgnomet. Chem., 1988, vol. 356, p. 151. doi https://doi.org/10.1016/0022-328X(88)83084-5
Tiekink, E.R.T., J. Organomet. Chem., 1987, vol. 333, p. 199. doi https://doi.org/10.1016/0022-328X(87)85152-5
Author information
Authors and Affiliations
Corresponding author
Additional information
Russian Text © V.V. Sharutin, O.K. Sharutina, A.N. Efremov, E.V. Artem’eva, 2019, published in Zhurnal Obshchei Khimii, 2019, Vol. 89, No. 1, pp. 89–94.
Rights and permissions
About this article
Cite this article
Sharutin, V.V., Sharutina, O.K., Efremov, A.N. et al. Synthesis and Structure of μ2-Oxobis(carboxylatotriarylantimony). Russ J Gen Chem 89, 76–81 (2019). https://doi.org/10.1134/S1070363219010146
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363219010146