Abstract
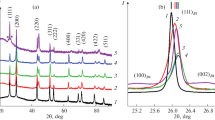
Thin nanostructured films of lead sulfide-based CdxPb1–xS solid solutions, strongly supersaturated with respect to the substituting component, were prepared by hydrochemical deposition from ammonia-citrate, ethylenediamine-citrate, and ethylenediamine reaction mixtures onto glass-ceramic substrates. The structure, composition, and morphology of the resulting films were examined. An interesting feature of the synthesis was revealed, a distinct correlation between the linear dimensions of the crystallites constituting the films and the cadmium sulfide content therein: the higher cadmium sulfide content, the smaller were the crystallites.
Similar content being viewed by others
References
Markov, V.F., Kitaev, G.A., Uimin, S.N., and Maskaeva, L.N., Innovatsii, 1998, no. 1, p. 55.
Maskaeva, L.N., Markov, V.F., Porkhachev, M.Yu., and Mokrousova, O.A., Pozharovzryvobezopasnost’, 2015, vol. 24, no. 9, p. 67.
Markov, V.F., Maskaeva, L.N., and Polikarpova, Yu.S., Butlerovsk. Soobshch., 2006, vol. 8, no. 1, p. 54.
Nichols, P.L., Liu, Zh., Yin, L., Turkdogan, S., Fan, F., and Ning, C.Z., Nano Lett., 2015, vol. 15, p. 909. doi 10.1021/nl503640x
Hernadez-Borja, J., Vorobiev, Y.V., and Ramirez-Bon, R., Sol. Energy Mater. Sol. Cells, 2011, vol. 95, p. 1882. doi 10.1016/j.solmat.2011.02.012
Caselli, D.A. and Ning, C.Z., Opt. Express., 2011, vol. 19, Suppl. 4, A686.
Obaid, A.S., Mahdi, M.A., Hasson, Z., and Bououdina, M., Superlatt. Microstruct., 2012, vol. 52, p. 816. doi 10.1016/j.spmi.2012.06.024
Barote, M.A., Kamble, S.S., Yadav, A.A., Suryavanshi, R.V., Deshmukh, L.P., and Masumdar, E.U., Mater. Lett., 2012, vol. 78, p. 113. doi 10.1016/j.matlet.2012.03.018
Calawa, A.R., Mrcoczkowski, J.A., and Harman, T.C., J. Electron. Mat., 1972, vol. 1, p. 191.
Shelimova, L.E., Tomashik, V.N., and Gritsyv, V.I., Diagrammy sostoyaniya v poluprovodnikovom materialovedenii (sistemy na osnove khal’kogenidov Si, Ge, Sn, Pb) [Phase Diagrams in Semiconductor Materials Science (Systems Based on Si, Ge, Sn, Pb Chalcogenides)], Moscow: Nauka, 1991.
Vesnin, Yu.I., Izv. Sib. Otd. Akad. Nauk SSSR, Ser. Khim. Nauk, 1987, vol. 17, no. 5, p. 145.
Vesnin, Yu.I., Izv. Sib. Otd. Akad. Nauk SSSR, Ser. Khim. Nauk, 1985, vol. 15, no. 5, p. 7.
Markov, V.F., Maskaeva, L.N., and Kitaev, G.A., Inorg. Mater., 2000, vol. 36, no. 12, p. 1194. doi 10.1023/A:1026613127267
Markov, V.F., Maskaeva, L.N., and Ivanov, P.N., Gidrokhimicheskoe osazhdenie plenok sul’fidov metallov: modelirovanie i eksperiment (Hydrochemical Deposition of Metal Sulfide Films: Modeling and Experiment), Yekaterinburg: Ural. Otd. Ross. Akad. Nauk, 2006.
Kirsanov, A.Yu., Markov, V.F., and Maskaeva, L.N., Vestn. Yuzhno-Ural’sk. Gos. Univ., Khim., 2013, vol. 5, no. 1, p. 35.
Maskaeva, L.N., Markov, V.F., and Gusev, A.I., Dokl. Phys. Chem., 2003, vol. 390, nos. 4–6, p. 147.
Pentia, E., Draghici, V., Sarau, G., Mereu, B., Pintilie, L., Sava, F., and Popescu, M., J. Electrochem. Soc., 2004, vol. 151, no. 11, p. G729. doi 10.1149/1.1800673
Tan, G.-L., Liu, L., and Wu, W., AIP Adv., 2014, vol. 4, p. 067107. doi 10.1063/1.4881878
Suryavanshi, K.E., Dhake, R.B., and Patil, A.M., Int. J. Adv. Sci. Tech. Res., 2014, vol. 2, no. 4, p. 858.
Osuwa, J.C., Oriaku, C.I., and Ezema, F.I., Chalcogenide Lett., 2009, vol. 6, no. 8, p. 385.
Montenegro, R. and Landfester, K., Langmuir, 2003, vol. 19, no. 15, p. 5996. doi 10.1021/la027019v
Wang, C.X. and Yang, G.W., Mater. Sci. Eng., 2005, vol. 49, no. 6, p. 157. doi 10.1016/j.mser.2005.06.002
Markov, V.F. and Maskaeva, L.N., Butlerovsk. Soobshch., 2011, vol. 24, no. 2, p. 33.
Adamson, A.W., Physical Chemistry of Surfaces, 3rd ed., New York: Wiley, 1976.
Tauson, V.L. and Abramovich, M.G., Fiziko-khimicheskie prevrashcheniya real’nykh kristallov v mineral’nykh sistemakh (Physicochemical Transformations of Real Crystals in Mineral Systems), Novosibirsk: Nauka, Sib. Otd., 1988.
Savvakin, G.I. and Trefilov, V.I., Dokl. Akad. Nauk SSSR, 1987, vol. 293, no. 1, p. 91.
Uvarov, N.F. and Boldyrev, V.V., Russ. Chem. Rev., 2001, vol. 70, no. 4, p. 265. doi 10.1070/RC2001v070n04ABEH000638
Khairutdinov, R.F., Russ. Chem. Rev., 1998, vol. 67, no. 2, p. 109. doi 10.1070/RC1998v067n02ABEH000339
Markov, V.F. and Maskaeva, L.N., Russ. Chem. Bull., 2014, vol. 63, no. 7, p. 1523. doi 10.1007/s11172-014-0630-7
Maskaeva, L.N., Fedorova, E.A., Shemyakina, A.I., Stepanovskikh, E.I., and Markov, V.F., Butlerovsk. Soobshch., 2014, vol. 37, no. 2, p. 1.
Katysheva, A.S., Markov, V.F., and Maskaeva, L.N., Russ. J. Inorg. Chem., 2013, vol. 58, no. 7, p. 833. doi 10.7868/s0044457x1307012x
Kitaigorodskii, A.I., Rentgenostrukturnyi analiz melkodispersnykh i amorfnykh tel (X-Ray Diffraction Analysis of Finely Dispersed and Amorphous Solids), Moscow: Gos. Izd. Tekh.-Teor. Lit., 1952.
Roduner, E., Nanoscopic Materials: Size-Dependent Phenomena, Cambridge: Royal Society of Chemistry, 2006.
Kumok, V.N., Kuleshova, O.M., and Karabin, L.A., Proizvedeniya rastvorimosti (Solubility Products), Novosibirsk: Nauka, 1983.
Kratkii spravochnik fiziko-khimicheskikh velichin (Concise Handbook of Physicochemical Quantities), Mishchenko, K.P. and Ravdel’, A.A., Eds., Leningrad: Kimiya, 1974.
Akhumov, E.I., Zh. Fiz. Khim., 1981, vol. 55, no. 12, p. 3038.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © L.N. Maskaeva, A.D. Kutyavina, V.F. Markov, I.V. Vaganova, V.I. Voronin, 2018, published in Zhurnal Obshchei Khimii, 2018, Vol. 88, No. 2, pp. 319–328.
Rights and permissions
About this article
Cite this article
Maskaeva, L.N., Kutyavina, A.D., Markov, V.F. et al. Features of the Formation of Thin Films of Supersaturated Cd x Pb1–xS Solid Solutions by Chemical Bath Deposition. Russ J Gen Chem 88, 295–304 (2018). https://doi.org/10.1134/S1070363218020172
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363218020172